Phoenix and Eos SENOLYTIX to present dual approaches to tackling aging at the 2nd Senotherapeutics Summit.
The second Senotherapeutics Summit in Rome will be a significant draw for anyone interested in the next frontier of longevity biotechnology. Senescent cells – damaged, non-dividing cells that accumulate with age and drive inflammation, tissue dysfunction and disease – remain one of the field’s most compelling targets. Removing or modulating these so-called ‘zombie cells’ has been shown in preclinical models to extend lifespan, improve organ function and even rejuvenate physical performance. For a sector still searching for its first validated, first-in-class therapy, senotherapeutics represent both the tantalising promise of healthspan extension and the formidable challenge of clinical translation.
That is why this year’s programme is attracting so much attention: Platinum sponsors Phoenix SENOLYTIX and Eos SENOLYTIX, both wholly owned sub entity ‘spokes’ of the holding company ‘hub’, SENOTHERAPEUTIX, Inc, are not only bringing data but staking out two different technological visions of what senotherapeutics can become. Founder and CEO Dr Kevin Slawin will give back-to-back presentations – first on Phoenix’s gene therapy platform ApoptiCIDe and then on Eos’ mitochondria-targeting peptide platform MitoXcel – offering the Rome audience a rare side-by-side view of strategies that could shape the next wave of longevity therapeutics.
Longevity.Technology: The senotherapeutics field has matured from elegant mouse models into a busy pipeline of competing technologies, each vying to claim the mantle of first safe and effective senolytic. What makes this Summit compelling is that it will showcase not just incremental progress but contrasting philosophies – one harnessing refined gene therapy vectors, the other leveraging mitochondria-targeting peptides. That both approaches are being advanced under the same leadership is unusual – and perhaps a recognition that eliminating senescent cells may require more than one arrow in the quiver.
Dr Kevin Slawin, Founder and CEO of both Phoenix and Eos SENOLYTIX, told Longevity.Technology that his team’s experience and ambition place them in a strong position to take on aging directly.
“My team and I, founders of Bellicum Pharmaceuticals, the first CAR T company that we took public on NASDAQ at a $500 million valuation in 2014, are no strangers to tackling the most difficult medical problems with innovative solutions,” he said. “The cost of health problems associated with aging are enormous and it has been said that a successful gerotherapeutic would have a value that is too high to calculate – it would instantly become the most valuable medical intervention in history because of the number of person-years of healthy life that could be manufactured.
“We believe that we have discovered just that, the first gerotherapeutics aimed at an aging-specific target, to extend healthspan and reduce healthcare burdens. I’m excited to share these discoveries for the first time with my colleagues at this meeting.”
Phoenix turns proof-of-concept into platform
Phoenix’s presentation will revisit the foundations of the field. The company has developed ApoptiCIDe-CE-GERO-002, a proprietary gene therapy vector carrying an iCasp9 ‘suicide’ switch under the control of a p16Ink4a-specific promoter. In naturally aged mice, this approach achieved 60–70% clearance of senescent splenic cells following administration of a proprietary dimerizer trigger. The work builds directly on the landmark p16Ink4a-ATTAC mouse model, created using Bellicum technology developed by Slawin’s prior company, which first demonstrated that periodic removal of senescent cells could delay tumorigenesis, attenuate organ decline and extend median lifespan. Phoenix now aims to refine this proof-of-concept into a therapeutic platform with the specificity and safety profile required for clinical trials.
Eos builds breadth through peptides
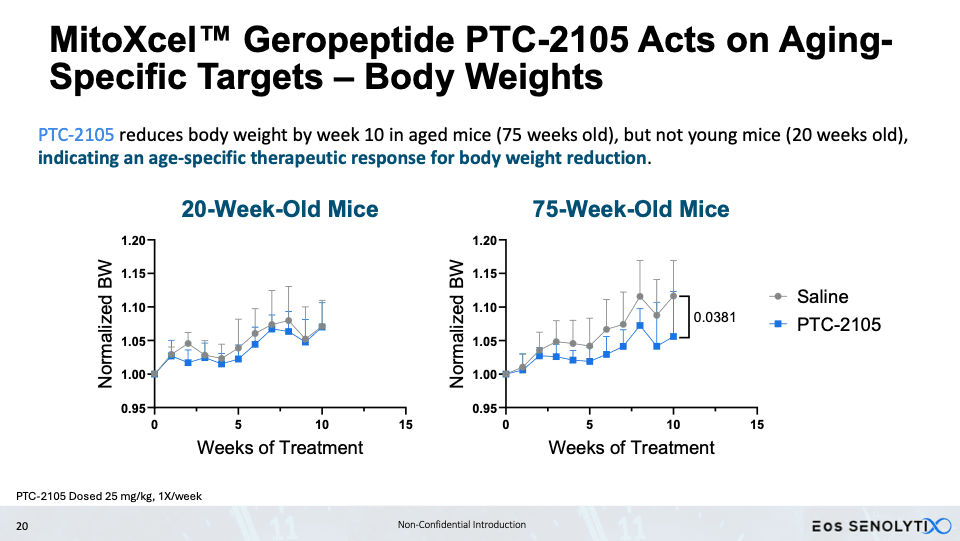
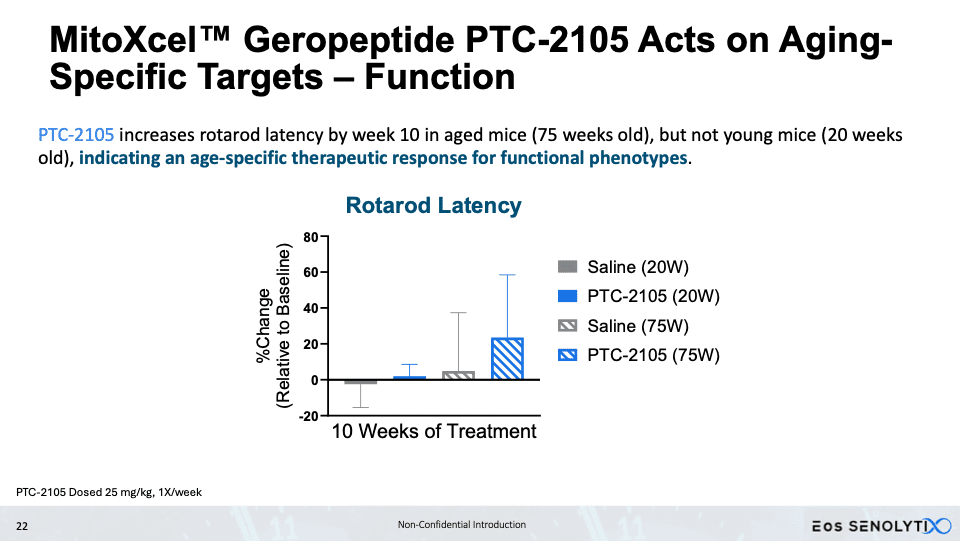
Eos, by contrast, is advancing a peptide-based strategy with its MitoXcel technology. These geropeptides target the lower mitochondrial membrane potential characteristic of aging cells, working through two complementary mechanisms: enhancing mitochondrial efficiency and inducing apoptosis in senescent cells. In aged mice, treatment with lead candidate PTC-2105 produced striking body composition changes – a 34% reduction in fat mass alongside a 23% increase in lean mass – accompanied by improvements in exercise endurance, grip strength and cognitive performance. Slawin’s team is also exploring oncology applications via a related company, Perseus SENOLYTIX, with early data in B-cell acute lymphoblastic leukemia suggesting broad therapeutic potential through the same mitochondrial-targeted mechanism. IND-enabling studies for Eos’s PTC-2105 are underway, with first-in-human trials planned for 2026.
Promise and pitfalls
The advantages of each approach are clear. ApoptiCIDe gene therapy offers precision – the ability to trigger apoptosis in cells expressing senescence markers – but also faces questions about delivery, safety and cost. MitoXcel geropeptide therapy appears to offer breadth and the potential for improvements in overall physical health and function, along with the added attraction of healthier weight loss benefits, differentiated from GLP-1 based therapeutics by a loss of fat but an increase in muscle mass, that resonate with current pharma priorities. Both approaches, however, must demonstrate safety and long-term tolerability; the effects on mitochondria and the resulting clearance of senescent cells must avoid collateral damage while delivering sustained functional gains.
Senotherapeutics, quo vadis?
Next steps will therefore be critical. For Phoenix, advancing ApoptiCIDe toward clinical translation means proving that gene therapy vectors can be targeted with the required fidelity and controlled safely in humans. For Eos, the challenge will be to convert compelling mouse data into reproducible human outcomes and to carve out clinical indications – from obesity in the elderly to oncology – where regulators and payers can be convinced of benefit. Both companies are raising capital to push their programmes forward, with Phase 1 trials in sight.
The Rome Summit will not provide definitive answers to these questions, but it will set the stage. With senotherapeutics edging closer to the clinic, and with two very different strategies being presented by the same CEO, delegates will see both the promise and the plurality of approaches that define this critical area of longevity science.
You can view the full Phoenix SENOLYTIX deck presented at the conference HERE and the Eos SENOLYTIX deck HERE.
Selected slides: