Confocal imaging allows scientists to study cellular structures and tissues with the help of pinhole apertures, optical sectioning, and image reconstruction.
©iStock, AndreasKermann
Since its inception, confocal microscopy has enabled scientists to visualize and analyze fixed and live cells, as well as subcellular structures, with greater precision and clarity than conventional microscopy. This article explores the history of confocal microscopy, as well as its working principles, types of confocal techniques, major experimental considerations, and wide-ranging confocal microscopy applications.
What Is Confocal Microscopy?
Confocal microscopy is a specialized fluorescence imaging technique that scientists use to acquire images at greater resolution than conventional microscopy.1 In addition to scanning the lateral x and y-axes, confocal microscopes collect thin optical sections along the vertical z-axis and sequentially arrange them to construct high-resolution, 3D images.2 Advances in computer processing, high-intensity lasers, sensitive photodetectors, and digital imaging software have further enhanced the resolution and expanded the applications of confocal microscopy.
History of confocal microscopy
In 1957, Marvin Minsky, a researcher at Massachusetts Institute of Technology (MIT), proposed a method to reject out-of-focus light during optical imaging, which significantly improved image quality. He patented this innovative concept, which led to confocal microscopy development.3 Almost a decade later, David Egger from Yale University and Mojmir Petran from Charles University built the first spinning disk, multiple-beam confocal microscope. In 1971 at Yale University, Paul Davidovits and David Egger developed the first mechanically scanned confocal laser microscope.
Initially, researchers used confocal microscopes to study the spatial distribution of one or two fluorescently labeled structures in living or fixed samples. However, recent advances have enabled them to distinguish between two closely spaced structures within a single organelle. For example, scientists now use high-resolution confocal microscopy to visualize the specific location of particular genes or sequences in nuclear chromosomes during in situ hybridization experiments.
Confocal microscopy versus conventional light microscopy
In traditional wide-field fluorescence microscopy, light from out-of-focus planes blurs the image, obscuring fine details.
In contrast, confocal microscopes use a point illumination source and a pinhole aperture to generate sharper images.4 Furthermore, in confocal microscopy, biospecimens are optically sectioned to eliminate physical sectioning artifacts. Optical sections have a relatively shallow depth of field, providing details of a single plane instead of the whole specimen.3
Lastly, conventional microscopy only captures images by scanning the x/y plane, whereas confocal microscopy also includes the z-axis for constructing detailed 3D images.3
How Confocal Microscopy Works
The main principle of confocal microscopy is to focus both the light source and the detector on one tiny spot in a sample at a time.5 A confocal microscope generates a complete image by scanning this focused spot of light across the sample and building the image point by point from the photons that pass through the pinhole to the detector. During confocal imaging, only the focused layer of the sample is clearly visible because the system blocks out-of-focus light.
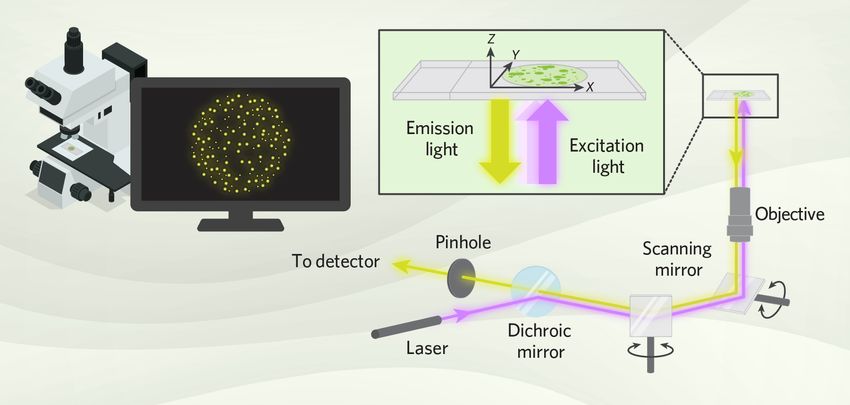
A confocal microscope scans a focused spot of excitation light across a sample and collects emitted fluorescence through a pinhole aperture, allowing researchers to build high-resolution 3D images point by point.
Modified from © istock.com, BoxerX, Golden Sikorka, Perkasa Rambe, Sai Than Lu; Designed by Ashleigh Campsall
Basic components of a confocal microscope
The key components of confocal microscopy and their functions include the following.5
- Lasers: Fluorescent light emission following laser-induced excitation forms the foundation of confocal microscopy. Scientists may use gas lasers (e.g., argon and helium-neon), fiber lasers, diode lasers, or solid-state lasers for their experiments. These light sources are more uniform, stable, and generate less heat than traditional light sources such as mercury and xenon lamps.
- Mirrors: Scanning mirrors rapidly move the laser beam across the sample, point by point, in both the x and y directions. Dichroic mirrors help separate the excitation light from the emission light.
- Objective lens: The objective lens focuses the laser beam to a specific focal plane or a diffraction-limited spot within the biospecimen and collects the emitted fluorescence. A lens with a higher numerical aperture (NA) typically produces higher-resolution and brighter images.
- Pinhole aperture: After fluorescence emission, the light passes through a spatial filter called a pinhole aperture that blocks any out-of-focus light in the sample. This step is crucial for eliminating background fluorescence emitted from deeper layers of the sample.
- Detectors: Sensitive light detectors, such as photomultiplier tubes (PMTs), convert the light signal to an electronic signal, allowing even a very weak fluorescence emission from the sample to be detected and measured accurately.
- Image processing system: The image processing system controls the microscope, synchronizes the scanning process, receives data from the detector, and processes it to create a 3D image of the sample.
Table 1: Practical considerations for designing confocal microscopy experiments5
|
Considerations |
Purpose |
|
Objective lens |
|
|
Sample preparation |
|
|
Depth penetration |
|
|
Fluorophore |
|
|
Pinhole size |
|
|
Imaging speed |
|
|
Laser power |
|
|
Calibration and alignment |
|
Confocal Microscope Types and Applications
Confocal microscopes come in different types, each designed for precise research needs and experimental conditions.
Laser scanning confocal microscopes
In a laser scanning confocal microscope (LSCM), scanning galvanometer mirrors move the laser beam in the x and y directions across the sample to generate an image of each optical section.5 Researchers change the focal point to collect a z-stack, acquiring images at different depths. After collecting all optical sections from top to bottom, they use digital software to reconstruct a 3D image of the sample.
The flexibility of LSCM enables researchers to perform fast imaging of dynamic processes in living cells, conduct detailed tissue morphology analyses, and study protein expression and localization patterns.6 For instance, scientists use LSCM extensively in reproductive biology research on animals and humans, particularly to assess oocyte quality.7
Spinning disk confocal microscopes
In contrast to LSCM, which scans one point at a time, the spinning disk confocal microscope (SDCM) simultaneously focuses on multiple points. SDCM utilizes a rapidly rotating metal disc with numerous fixed-width pinholes, arranged in outwardly spiraling tracks.5 As the disk spins at high speed, the pinholes scan across the sample in parallel rows, quickly building up an image. This parallel scanning greatly increases the speed of image acquisition and reduces photodamage.
Cell biologists rely on SDCMs to study living cell dynamics, intracellular processes, and protein interactions.8 In a recent study, scientists employed SDCM to investigate mitochondrial quantity, morphology, and membrane potential in living human multipotent stem cells associated with blood vessels.9
Hybrid scanning confocal microscopes
A hybrid scanning confocal microscope (HSCM) combines the strengths of LSCMs and SDCMs.5 This system replaces the round pinhole with a rectangular slit to block out-of-focus light. By using a slit-scanning system, researchers can cover a larger portion of the sample in each field of view, thereby accelerating imaging speed. However, this approach can increase the risk of photodamage due to rapid photobleaching and may result in lower resolution.
The swept-field confocal microscope (SFC) is a hybrid system that utilizes either a pinhole or a slit scanning mode, where the apertures remain stationary and the scanning mirrors and galvanometer scan the image of the illuminated apertures across the sample.10 Researchers focus the emitted photons through a complementary set of pinholes or slits onto a detector called a Charge-Coupled Device (CCD) camera. This approach offers high-speed imaging, improved light collection efficiency, and reduced artifacts that are typically generated by moving apertures. Researchers primarily implement swept-field confocal microscopy for live-cell imaging at high spatial and temporal resolutions.
Reflectance confocal microscopes
A reflectance confocal microscope (RCM) is a novel technology that sets the emission wavelength to overlap with the excitation beam wavelength, allowing the detection of reflected laser signals.11 This technique does not rely on fluorescent dyes, which is a key advantage. However, optical interference effects can occur and require careful interpretation.
Researchers primarily use RCMs to noninvasively examine skin with nearly histologic resolution. By combining RCM methods with dermoscopy, clinicians have significantly improved skin cancer diagnosis and reduced the number of biopsies performed on benign skin lesions.12
Future Confocal Microscopy Outlooks
Continual advances in confocal microscopy provide improvements in image resolution, speed, and sensitivity. Researchers are integrating super-resolution techniques, such as stimulated emission depletion (STED) microscopy and structured illumination microscopy (SIM), with confocal systems to surpass the diffraction limit and visualize cellular structures at the nanoscale.13 They are also applying advanced computational methods, including deconvolution algorithms and artificial intelligence-driven image reconstruction, to enhance image resolution, contrast, and quantitative analysis.14 These advances will enable scientists to observe dynamic molecular interactions and organelle architecture in unprecedented detail, opening new avenues for understanding complex biological processes.
FAQ
What is confocal microscopy?
- Confocal microscopy is an advanced optical technique that produces high-resolution 3D images. Scientists commonly use this microscopy method to examine cell structure and investigate cellular dynamics and interactions within living or fixed cells and tissues.
What is a unique feature of confocal microscopy?
- Confocal microscopy provides clear images from a thin section of a thick sample with minimal interference by employing a pinhole spatial filter to eliminate out-of-focus light, resulting in high-contrast images.
How are samples stained for confocal microscopy?
- In confocal microscopy, scientists label the target molecules or structures with fluorescent dyes, known as fluorochromes. They employ various staining methods, including direct labeling and indirect labeling. In the direct method, the fluorescently labeled molecule directly binds to the target. In contrast, the indirect method uses a primary antibody that binds to the target, and subsequently, a fluorescently labeled secondary antibody binds to the primary antibody.
- Peterson DA. Confocal microscopy. In: Encyclopedia of Movement Disorders. Academic Press; 2010:250-252.
- Zhang J, et al. Optical sectioning methods in three-dimensional bioimaging. Light Sci Appl. 2025;14(1):1-28.
- Wright SJ, Wright DJ. Introduction to confocal microscopy. Methods Cell Biol. 2022;70:1-85.
- Fouquet C, et al. Improving axial resolution in confocal microscopy with new high refractive index mounting media. PLoS One. 2015;10(3):e0121096.
- Elliott AD. Confocal microscopy: Principles and modern practices. Curr Protoc Cytom. 2020;92(1):e68.
- Bayguinov PO, et al. Modern laser scanning confocal microscopy. Curr Protoc Cytom. 2018;85(1):e39.
- Folts L, et al. Tissue clearing and imaging approaches for in toto analysis of the reproductive system.Biol Reprod. 2024 Jun;110(6):1041-1054.
- Stehbens S, et al. Imaging intracellular protein dynamics by spinning disk confocal microscopy.Methods Enzymol. 2012;504:293-313.
- Ahmadian S, et al. Spinning disk confocal microscopy for optimized and quantified live imaging of 3D mitochondrial network. Int J Mol Sci. 2024;25:4819.
- Castellano-Muñoz M, et al. Swept field laser confocal microscopy for enhanced spatial and temporal resolution in live-cell imaging.Microsc Microanal. 2012;18(4):753-60.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4(10):1014-1023.
- Faldetta C, et al. Melanoma clinicopathological groups characterized and compared with dermoscopy and reflectance confocal microscopy. J Am Acad Dermatol. 2024;90(2):309-318.
- Valli J, et al. Seeing beyond the limit: A guide to choosing the right super-resolution microscopy technique. J Biol Chem. 2021;297(1):100791.
- Tian W, Chen R, Chen L. Computational super-resolution: An odyssey in harnessing priors to enhance optical microscopy resolution.Anal Chem. 2025;97(9):4763-4792.