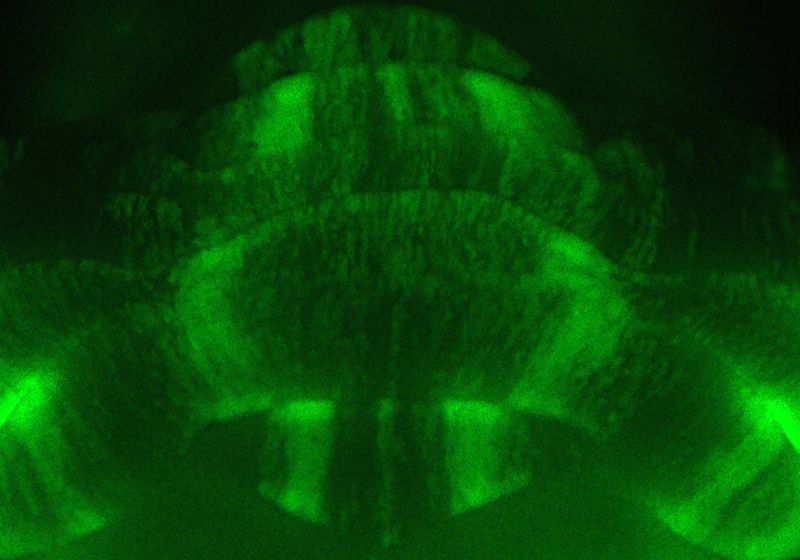
As the brain ages, it loses cells. Researchers have predominantly focused on the effects of cell death in brain areas responsible for cognition, but the cerebellum, most well-known for coordinating motor function, has emerged as an important player in the aging brain too.1 The primary cell type in the cerebellum, the Purkinje cell, degenerates even in healthy individuals.2,3 However, this process and its role in aging are understudied.
In a reviewed preprint in eLife, researchers from Baylor College of Medicine demonstrated that otherwise healthy but aged mice experience neurodegeneration of Purkinje cells in a consistent, striped pattern.4 The findings provide a roadmap to studying the mechanisms of aging and the effects of neurodegeneration on cognitive and motor function.
Sarah Donofrio found a novel pattern of cell death in aged mouse cerebella.
Lauren Peacoe
“[The study] actually came about in a really unexpected way,” said Sarah Donofrio, a study coauthor and a graduate student researching the effects of lost Purkinje cells on tremor. As part of her research, she had been practicing a staining technique using brains from mice that had aged out of the group’s preferred window over the course of pandemic closures.
When she looked at her work, she at first thought that she had made a mistake because several regions of the mouse brains seemed to show missing cells. Looking at the stained tissue, her advisor, neuroscientist Roy Sillitoe, told her, “No, Sarah, these are stripes. You found something incredible,” she recalled.
Instead of Purkinje cells dying homogenously across the cerebellum, as was thought to happen, the team saw that the cells died in what appeared to be consistent striped patterns in the aged mice.
“In development, patterns are the source of how you understand gene expression, how you understand where cells are born, [and] where they end up migrating to,” Sillitoe said. He previously studied patterns in embryogenesis, and when he saw the stripes in Donofrio’s samples, he said, “Wait a minute, there’s patterns on the other side of life as well.”
The team launched into characterizing the patterns. To study the intact cerebellum, they used wholemount immunohistochemistry and light sheet microscopy. They saw that, although not every aged mouse experienced cell degeneration, when the striping occurred, its pattern was consistent and progressive. They used histology and fluorescent reporter mice to confirm that the Purkinje cells underwent neurodegeneration and death as opposed to altering their expression of surface markers.
“What I wanted to know is whether the pattern that I saw on aged mice was similar to or different from some of these known [disease-associated] patterns,” Donofrio said. To do this, the team looked at one cerebellar marker that defines neurodegeneration in several mouse models. Although cell death in one region of the cerebellum coincided with this marker, not all regions followed this trend, supporting the hypothesis that the observed pattern was unique to the aging brain.
“I was really impressed reading through the methodology, with the ways in which they looked at this in a very thoughtful manner,” said Jessica Bernard, a cognitive neuroscientist at Texas A&M University who was not involved with the study.
“You see this happening, not just kind of all over, but there’s a pattern to the cell loss, which is really interesting,” said Bernard. She said that although the current study didn’t explore what caused this, “that’s okay, because it gives us something really interesting to look at and try to figure out and understand why.”
To determine whether or not these patterns influenced motor function decline in aging mice, the team assessed the capacity of young and aged mice to perform two different movement tests. They also studied the degree of tremor in the animals.

Roy Sillitoe studies the role of Purkinje cell dysfunction in motor disorders. He previously studied developmental patterns in embryonic development.
Lauren Peacoe
The team showed that aged mice had decreased motor behavior and greater tremor frequency compared to young mice. However, when they assessed the older animals’ cerebella, they did not see a relationship between decreased motor behavior and striping from lost Purkinje cells. Bernard said that studying how cognitive behaviors relate to the striping pattern in future research could also be interesting.
Finally, the team looked at cerebellum sections from postmortem brains of a young, middle-aged, and older human, none of whom had reported neurological damage at their time of death. “We were able to visualize the human Purkinje cells really beautifully, and even more excitingly is we could see that there was really extensive Purkinje cell death in this [older] human patient,” Donofrio said.
Because they only had sections of the tissue but did not know which region of the cerebellum it came from, the team could only conclude that they saw a loss of cells in the older patient, which reflects what they saw in aged mouse brains. This cell loss was absent in the younger brain. “Is it going to be a pattern? Almost certainly should be, but we don’t know that for certain,” Sillitoe said, adding that he found similar shared patterns in his work studying development patterns between different species and is optimistic that a similar trend will hold true for degeneration.
Bernard agreed that the study provides a jumping off point for more human studies. “The other thing that will be interesting is considering what this means in age-related neurodegenerative disease,” she said.
Similarly, Donofrio thought the findings could help researchers better understand aging biology. “Aging is incredibly complex, and there are very organized processes that may be going on underneath the surface, where neurodegeneration is kind of chaotic. You have different cells dying at different times in different ways, but there may be an underlying pattern that dictates how and when and why these cells are dying.”
Sillitoe agreed, comparing it to having a map. “These patterns, which I once thought of as just a beautiful developmental platform, 25 years later in my life, almost 30 years, have this potential to give us a therapeutic map.”