University of Oregon (UO) researchers have tested a new combination drug therapy that experimental results suggest could dismantle the difficult-to-treat bacteria inhabiting chronic wound infections. The findings could help lead to the development of more effective antimicrobial treatments that promote healing in chronic wounds. Such treatments might also help to reduce the risk of severe infections, such as diabetic foot ulcers, that may lead to amputations.
The approach pairs long-known drugs that do little on their own against the hard-to-treat bacterial pathogen Pseudomonas aeruginosa festering in chronic wounds. Headed by Melanie Spero, PhD, an assistant professor of biology in the UO’s College of Arts and Sciences, the researchers found that combining antibiotic therapy with small doses of chlorate proved 10,000 times more effective at killing bacterial cells in the lab than single-drug antibiotics.
That level of potency also reduced the dose of medication required to kill P. aeruginosa. And while the research was carried out in the context of chronic wound infections, the strategy may hold promise for addressing antibiotic resistance more broadly. “I think that drug combinations will be a critical approach that helps us fight against the rise of antibiotic resistance,” Spero said. “Finding examples of synergy among antimicrobials that are already on the market is going to be really valuable. And we’ll need to dig further into the mechanisms behind why they work well together.”
If the findings can be translated to humans, they could help shorten the time patients need to be on antibiotics and lower the risks of toxicity, added Spero, who is senior author of the team’s published study in Applied and Environmental Microbiology, titled “Hijacking anaerobic metabolism to restore antibiotic efficacy in Pseudomonas aeruginosa,” in which they concluded, “Our results demonstrate that combined chlorate-antibiotic treatment holds promise for combating antibiotic treatment failure in hypoxic host environments.”
A chronic wound is injured tissue that hasn’t started to heal within normal time frames of four to 12 weeks. The most common type is a diabetic foot ulcer, Spero said, which is an open sore on the foot’s underside that forms from poor circulation, prolonged pressure, and a lack of sensation.
According to research published by the American Diabetes Association, about 1 in 4 people with type 2 diabetes develop a foot ulcer, and more than half of those cases become infected. “An active infection is the most common complication that prevents the wound from healing and closing,” Spero said, adding that when severe, 1 in 5 diabetic foot ulcers require an amputation. “It’s very debilitating, but there’s not a lot of microbiology research being done in this field. So it’s an opportunity to make a big difference.”
Shifts in blood flow, the high oxygen demand of inflammatory cells, and the presence of bacteria in the chronic wound site all limit how much oxygen reaches the tissue, preventing healing. “Bacterial pathogens routinely encounter hypoxic and anoxic microenvironments within the human body,” the authors wrote in their paper. “In chronic wounds, which affect ~2% of the U.S. population, tissue hypoxia stems from insufficient blood supply as well as local O2 consumption by microbes and overactive immune cells.”
Those low-oxygen conditions are also the very problem that makes bacterial infections hard to fight: They unmask antibiotic resistance and tolerance. “Many antibiotics are less effective at killing pathogens under oxygen (O2)-limited conditions,” the team continued. “The relationship between environmental hypoxia and antibiotic treatment failure is thought to underpin different types of recalcitrant infections, including chronic wound and cystic fibrosis (CF) airway infections.” Chronic wounds also cannot heal while there is an active infection, and the frequent failure of antibiotic treatments to resolve wound infections can lead to complications such as limb amputation.
When a wound site becomes oxygen-limited, bacteria can switch to nitrate respiration for energy, the scientists further explained. And while bacterial growth slows without oxygen, the bacteria still survive and continue to spread. “Nitrate respiration is a widespread form of anaerobic energy metabolism that supports the growth or survival of many pathogens in hypoxic host environments,” the authors wrote.
The resulting slow growth of the bacteria, particularly P. aeruginosa, makes them notoriously tolerant to conventional antibiotics. That’s because many medications are rated based on how well they kill fast-growing bacteria, Spero said. But if the bacteria are growing slowly, those antibiotics, which are also often tested only in oxygen-rich conditions, end up being ineffective, she said. At least when administered on their own, Spero and colleagues have now found. When the antibiotics are combined with the small molecule called chlorate, it “stresses the bacterial cell in a way that makes it super susceptible to antibiotics,” Spero said.
During nitrate respiration, nitrate reductase reduces nitrate (NO3−) to nitrite (NO2−). And while there are several types of nitrate reductase, there is “strong evidence”, the team continued, that nitrate respiration mediated by the enzyme Nar supports pathogen survival or growth in the host. “… we recently showed that the opportunistic pathogen Pseudomonas aeruginosa requires Nar to cause persistent chronic wound infections in diabetic mice.” The findings from multiple studies taken together, the team continued, indicate that “… nitrate respiration is a promising therapeutic target for killing pathogens in hypoxic or anoxic host environments.”
The newly reported research builds on studies Spero first conducted as a postdoctoral scholar at the California Institute of Technology. She previously found that chlorate, a simple compound that is harmless to mammals and humans in the low doses used in her studies, turns antibiotics from lukewarm performers into potent bacteria killers in cell cultures and diabetic mouse models.
“It has been known for decades that chlorate is toxic to bacteria that respire nitrate via Nar because this enzyme reduces chlorate to generate the highly toxic chlorite molecule,” the team continued in their newly released report. “Chlorate (ClO3−) is a nitrate analog that acts as a prodrug: chlorate itself is relatively nontoxic, but Nar can bind and reduce chlorate to generate chlorite (ClO2−), which is a toxic oxidizing agent.” Mammals lack Nar, so it is “unsurprising” that chlorate has low toxicity in mammals, they added.
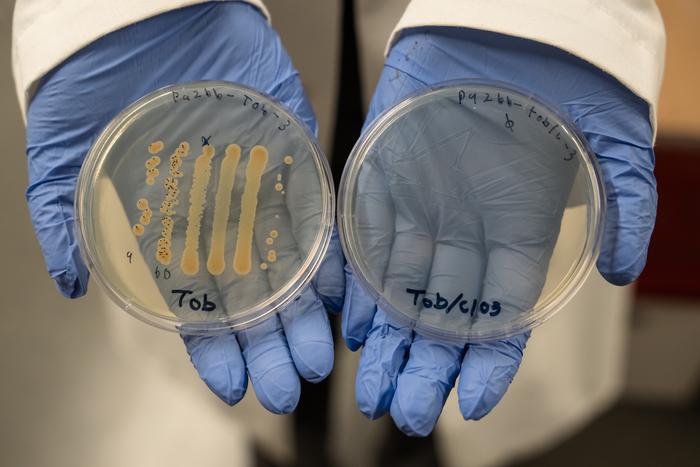
Through their latest study, Spero and colleagues showed that chlorate works to make all kinds of antibiotics more effective at killing P. aeruginosa, and can lower the antibiotic dose needed to fight the pathogen. For their research, they focused on different classes of anti-pseudomonal antibiotics, including aminoglycosides (tobramycin), fluoroquinolones (ciprofloxacin), beta-lactams (cephalosporins: ceftazidime), and polymyxins (colistin).
Their results showed that with a small amount of chlorate in the mix, it was possible to use just 1% of the standard dose of the broad-spectrum antibiotic ceftazidime. “… we found that chlorate potentiates all tested antibiotics,” they stated. And despite chlorate-only treatment resulting in little-to-no killing of hypoxic P. aeruginosa cultures, they pointed out, the addition of chlorate to each antibiotic treatment increased killing by more than four orders of magnitude for all tested classes of antibiotics. “In the case of ceftazidime, chlorate addition reduced the toxic dose by >100-fold.”
Promisingly, the investigators also reported that chlorate potentiated antibiotic therapy against all P. aeruginosa clinical isolates tested. “Overall, chlorate-antibiotic combinations overcome antibiotic recalcitrance to kill various P. aeruginosa isolates under O2-limited conditions,” they stated. Spero added, “In the case of chronic infections, people are often on antibiotics for long periods of time, and that can wreak havoc on the body,” Spero said. “Drugs with high toxicities can disrupt gut microbes and have severe side effects. Anything we can do to shorten the amount of time that a person is going to be on antibiotics and lower the dosage, the better.”
The results come from controlled lab tests on bacterial cell cultures, so translation to the clinic is still far down the line. Especially since chronic infections usually don’t involve a single bacterium, Spero commented, as they host whole microbial neighborhoods living and interacting together. So uncovering how drug combinations affect those complex communities in model organisms is an obvious next step, she added. Nevertheless, the authors wrote, “These findings underscore the potential for chlorate to enhance the efficacy of antibiotic treatments in environments that are typically characterized by antibiotic recalcitrance.”
The exact mechanism for how chlorate boosts antibiotics is also still a mystery. Spero explained that chlorate has been known by scientists to hijack nitrate respiration, so in the complete absence of oxygen, microbes are wiped out. But in microenvironments of low—or high—levels of oxygen, the bacteria can somehow repair that damage and tolerate the chemical. So, in traditional single-drug screenings, which are usually performed in high-oxygen conditions, chlorate has been overlooked, Spero noted.
“I think that’s what we don’t fully appreciate: the types of stresses these compounds impose on the cell that are invisible to us,” she said. “If our only metric is viability—did the bacteria live or die?—that’s all we’ll look for. We need to be asking what processes are being pushed on or stressed out in the cell that can lead to its collapse in the presence of antibiotics.”
Spero hopes that looking “under the hood” of a cell during chlorate-antibiotic exposure will show scientists the biological machinery of how bacteria become susceptible to a range of antibiotics. “Chlorate is a promising candidate for pursuing future studies to uncover mechanisms of drug potentiation because it enhances killing in combination with drugs that have different mechanisms of action,” the team pointed out in their paper.
“This will have important implications not only for treating chronic wound infections but also broadly for the infectious disease field and our fight against antibiotic resistance and treatment failure,” Spero commented. “Once we understand the mechanisms of drug synergy, we can start to find other molecules that elicit these synergistic behaviors, and it won’t feel like a guessing game where we test every possible drug combination. We can start doing rational drug design, using molecules that have already been approved.”
The authors further commented, “Ultimately, the cellular stresses that drugs impose individually and in combination will begin to illuminate mechanisms of drug synergy, which will enhance our ability to predict powerful new drug combinations in the fight against antibiotic treatment failure.”