Immunoassays help researchers visualize important biological elements, allowing them to judge the relationship or quantities of specific antigens.
©iStock, Wirestock
Scientists rely on immunoassays in diverse settings, from basic research to clinical diagnostics and biopharmaceutical analysis.1 This article explores immunoassay principles, types, applications, and emerging trends that define modern laboratory practices.
What Is an Immunoassay?
Immunoassays are measurement procedures that use immune-based reagents to generate a detectable signal, allowing scientists to quantify biological processes. Scientists perform immunoassays to measure biological targets, pathway activity, and cellular phenotypes.2
To label targets, immunoassays depend on the interaction between an antibody and its target antigen’s binding site, known as an epitope.3 Antigen-antibody binding specificity ensures recognition of the intended molecule, while affinity and avidity determine the strength and stability of the interaction.1,4
Antibodies, also known as immunoglobulins, provide selectivity and sensitivity for immunoassay development.3 Scientists develop and screen antibodies to ensure they have the binding properties required to recognize target molecules. These properties ensure that the presence of a target molecule produces a reliable and detectable signal. Depending on the assay format, enzymatic, fluorescent, luminescent, or electrochemical labels display measurable signals. Assay quality relies on signal robustness and reproducibility, which in turn depends on optimized reagents, reaction conditions, instrumentation, and data analysis.2
Major Immunoassay Types
Over the last several decades, scientists have developed a wide range of immunoassay formats. Choosing among these approaches requires balancing biologically relevant signal detection with the need for robust, high-throughput data.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) are the most widely applied immunoassay format, providing high sensitivity, specificity, and throughput.1 These plate-based assays allow researchers to detect antigens in liquid samples using enzyme, chemiluminescent, or fluorescent labels. ELISA formats include sandwich and competitive designs, and many laboratories now employ automated or rapid one-step versions to increase efficiency.1,3
Flow cytometry
Scientists use flow cytometry to study cells and other particles in liquid suspension. By tagging different target antigens with unique fluorochrome-labeled monoclonal antibodies, they can simultaneously measure multiple markers within a single sample, generating detailed multiparameter profiles.3
Western blotting
In western blotting, or immunoblotting, scientists first separate proteins by properties such as size and charge using electrophoresis and then transfer the proteins to a membrane. Researchers next detect specific target proteins using enzyme-linked antibodies that generate chemiluminescent signals.3
Immunoprecipitation
Immunoprecipitation isolates target proteins or nucleic acids from liquid samples by forming antigen-antibody complexes, allowing researchers to study protein interactions or prepare purified proteins for further analysis.3,5
Lateral flow immunoassay
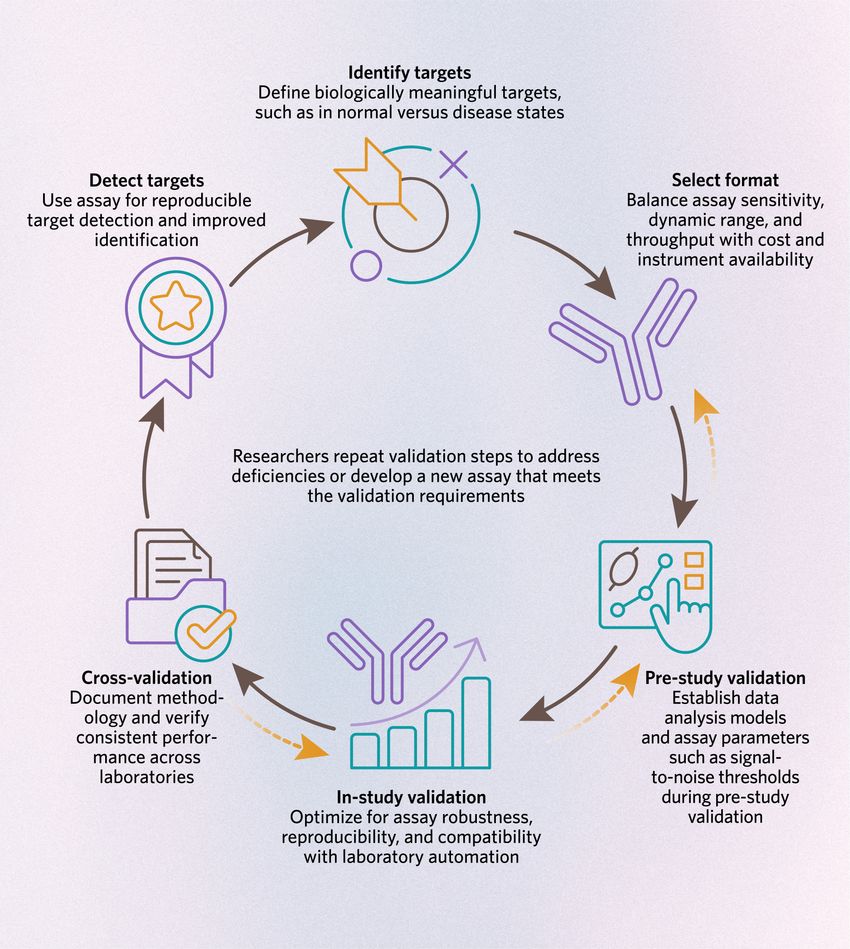
Immunoassay development is iterative, with each step informing one another to enable reproducible and meaningful target identification.
modified from © istock.com, bsd studio
Scientists use lateral flow immunoassays (LFIA) as simple, point-of-care devices to rapidly detect the presence or absence of analytes in body fluids, such as home pregnancy tests. These assays are widely adopted because they are low-cost, easy-to-use, and they deliver results within minutes without the need for specialized instrumentation.3,4 Advances in microfluidics and lab-on-a-chip technologies have further enhanced LFIA performance, enabling automated formats that require minimal reagent volumes and support remote semi-quantitative analyte measurements.1
Multiplex immunoassay technologies
Researchers use multiplex immunoassays or panels to measure multiple analytes simultaneously from a single low-volume sample for different analyses. Physicians increasingly rely on the clinical laboratory reports generated from multiplex immunoassays to inform comprehensive therapeutic assessments.1
Immunoassay Applications
Their ease of use, rapid turnaround, and high specificity make immunoassays valuable across laboratory and clinical settings, and scientists increasingly deploy them in point-of-care environments.
Clinical immunoassays
Immunoassays support clinical decision making by enabling healthcare professionals to measure the disease biomarkers that guide diagnosis and therapeutic monitoring.1 An immunoassay’s precision and sensitivity contributes to its indispensability for early detection and rapid screening.4
In addition to single-analyte tests, multiplex immunoassay platforms allow technicians to simultaneously detect multiple biomarkers, monitoring metabolic, lipid, liver, renal, and cardiac indices. They provide healthcare teams with clinically useful profiles that support disease scoring systems and condition severity assessment. To ensure reliability in healthcare settings, all immunoassays must undergo rigorous clinical validation before routine use.1
Infectious disease diagnostics
Physicians primarily diagnose many infectious diseases by measuring biomarker concentrations via immunoassays.1 For infections such as Lyme disease, cryptococcal meningitis, and syphilis, immunoassays that detect antibodies or antigens in blood or cerebrospinal fluid remain the standard method of confirmation.6
Although the World Health Organization has recommended PCR as the preferred COVID-19 screening tool, immunoassays have also been essential for public health initiatives, case identification in resource-limited settings, retrospective epidemiological surveys, and evaluating vaccine effectiveness.7
Commonly quantified biomarkers
Oncologists routinely employ immunoassays to measure plasma tumor markers such as prostate specific antigen (PSA), cancer antigen 125 (CA125), and alpha fetoprotein (AFP) for early detection and monitoring in at risk populations.8
Cardiac troponin-specific immunoassays are the gold standard for detecting myocardial injury, with fluorescence-based formats offering cardiologists rapid and highly sensitive results. Strategies such as tracking biomarker dynamics over time or combining immunoassays with imaging modalities have improved the diagnostic value of many biomarkers.8
Biopharmaceutical development and quality control
Immunoassays allow biopharmaceutical scientists to reliably detect a wide range of analytes across discovery, development, and manufacturing.9 For instance, researchers rely on immunoassays to monitor biologics, antibody titers, and potential contaminants, ensuring product safety and consistency.1
Automated ELISA formats remain the standard for many quality control applications, while real-time surface plasmon resonance-based assays are gaining adoption for their sensitive, label-free analysis.1
Advancements and Trends in Immunoassays
Scientists advance immunoassay technologies toward faster, more portable, and more sensitive formats. Point-of-care systems help deliver testing outside centralized laboratories. Lab-on-a-chip assays are commercially successful in clinical diagnostics, and microfluidics-based designs enable multiplexed, miniaturized, and high-throughput testing. Recently, more patients adopt smartphone-integrated devices for personalized healthcare monitoring, though further validation is needed before broad clinical accreditation.1
Electrochemical immunosensors represent another fast-growing frontier, transforming antigen-antibody binding into electrical signals for highly sensitive, low-cost disease biomarker detection.10,11 The future of immunoassays is increasingly decentralized, integrated into digital health platforms, and capable of providing precise, multiparameter data at the point of need.
Future Immunoassay Outlooks
Immunoassays remain indispensable tools for precise biomarker detection across healthcare and biopharmaceutical development.6 As technologies move toward miniaturization, multiplexing, and integration with digital platforms, scientists expand their reach beyond central laboratories. Future efforts will focus on improving sensitivity and accessibility, ensuring that immunoassays continue to provide cost-effective and reliable solutions across disciplines.
- Vashist SK, et al. Handbook of immunoassay technologies: Approaches, performances, and applications. Elsevier; 2025.
- Cox KL et al. Immunoassay methods. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2019.
- Gao Y, et al. A brief review of monoclonal antibody technology and its representative applications in immunoassays. J of Immunoassay and Immunochem. 2018;39(4):351-364.
- Di Nardo F, et al. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors (Basel). 2021;21(15):5185
- Nelson JD, et al. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34(1):e2.
- Theel ES, et al. Immunoassays for diagnosis of infectious diseases. M of Clin Microbio. 2015:91-105.
- Wang D, et al. Recent advances in immunoassay technologies for the detection of human coronavirus infections. Front Cell Infect Microbiol. 2022;12:1040248.
- Meany DL, et al. Early detection of cancer: Immunoassays for plasma tumor markers. Expert Opin Med Diagn. 2009;3(6):597-605.
- Darwish IA. Immunoassay methods and their applications in pharmaceutical analysis: Basic methodology and recent advances. Int J Biomed Sci. 2006;2(3):217-235.
- Chen H, et al. The applications of electrochemical immunosensors in the detection of disease biomarkers: A review. Molecules. 2023;28(8):3605.
- Puiu M, et al. Early detection of tumour-associated antigens: Assessment of point-of-care electrochemical immunoassays. TrAC Trends in Analytical Chemistry. 2023;160:116981.