Company plots Phase 3 trial of drug that targets a unique metabolic switch to ‘reawaken’ inactive stem cells and stimulate hair growth.
Regenerative medicine company Pelage Pharmaceuticals has raised $120 million in a Series B financing to advance its hair loss drug into late-stage clinical testing. The company plans to use the proceeds to support Phase 3 studies of PP405, a topical small molecule designed to reactivate dormant hair follicle stem cells and restore natural hair growth in men and women with androgenetic alopecia.
Androgenetic alopecia, commonly known as male or female pattern hair loss, is the most prevalent form of hair loss, affecting more than 80% of men and up to 40% of women over their lifetimes. The progressive, age-associated condition is driven by a combination of genetic and hormonal factors that cause gradual miniaturization of hair follicles, shortening of the hair growth cycle, and eventual loss of visible hair.
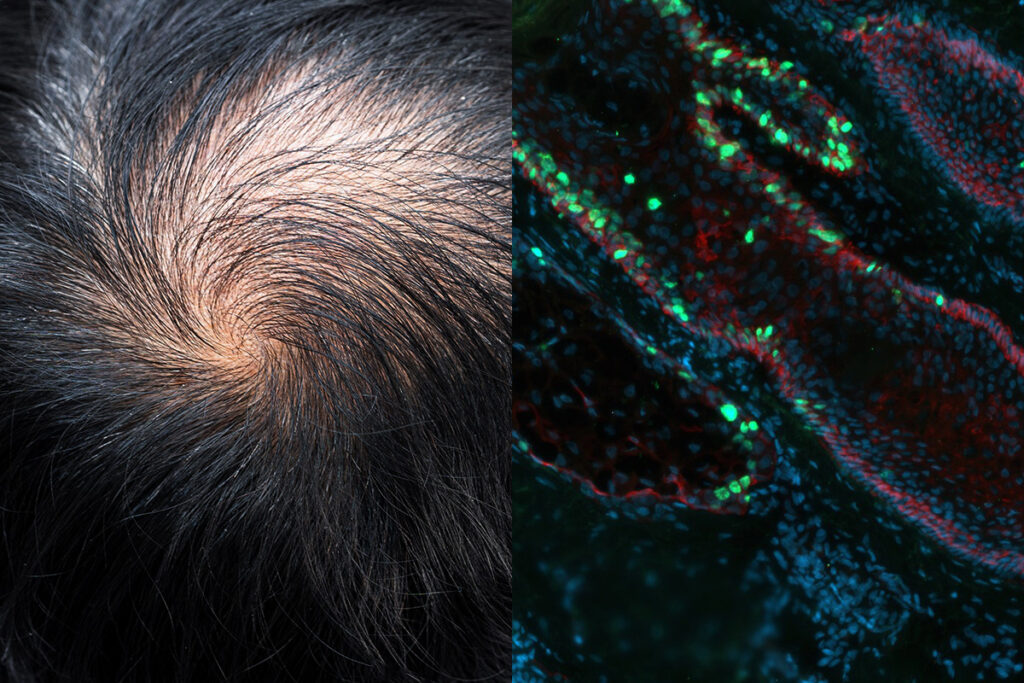
Los Angeles-based Pelage was founded based on discoveries made at UCLA identifying a unique metabolic switch in hair follicle stem cells. PP405, an MPC inhibitor, targets this switch to reawaken dormant stem cells and reinitiate the hair growth cycle. The company believes this distinguishes its approach from currently available hair loss treatments, which largely focus on hormonal or vascular pathways rather than direct stem cell activation.
Pelage CEO Dr Daniel Gil said that innovation in the hair loss field “has been lacking and a new FDA-approved option has not become available for decades.”
“This new funding from leading investors reinforces not only the need in the space, but the quality of our scientific approach and clinical results to date, which are enabling us to enter Phase 3 trials for lead program PP405 in 2026,” he said.
Interim results from a randomized, placebo-controlled Phase 2a trial reported in mid-2025 showed that PP405 met its primary safety endpoint and was well tolerated in both men and women with androgenetic alopecia. While the trial was designed to assess safety rather than efficacy, early signals indicated biological activity. Four weeks after dosing ended, 31 percent of men with more advanced hair loss who received PP405 achieved greater than a 20 percent increase in hair density, compared with none in the placebo group. The treatment also appeared to induce new hair growth from previously inactive follicles, offering preliminary evidence of regenerative potential.
“Our Phase 2a results show translation of the basic mechanism of stem cell reactivation to the human scalp, expanding the possibilities of regenerative medicine and validating a stem cell-directed approach in treating age-related conditions,” a spokesperson for Pelage told us.
Following completion of the controlled phase, participants initially randomized to placebo were offered enrollment in a three-month open-label extension to evaluate longer-term safety, which has now concluded. Pelage plans to present full Phase 2a data next year before initiating its Phase 3 trials.
The financing round follows last year’s $14 million Series A extension, and was co-led by ARCH Venture Partners and GV (Google Ventures), with participation from Main Street Advisors, Visionary Ventures, and YK Bioventures. Cathy Friedman of GV has been appointed Chair of the company’s board, with Richard Heyman of ARCH Venture Partners also joining as a director.
“Pelage is a pioneer in the regenerative medicine space, with a scientifically rigorous approach to address hair loss that sets them apart from currently available treatments,” said Friedman. “This could fulfill an urgent need, as we see a growing portion of the population experiencing hair loss, including women, who only have one FDA-approved option.”
Photograph: chormail/Envato