Modifying the progression of metabolic, immune, and age-related diseases through the gut microbiome
The development of the gut microbiome and alterations linked to health and disease across the lifespan was the focus of a symposium. Cristina Campoy, PhD, MD, from the University of Granada, covered the association between the developmental trajectory of the healthy human gut microbiome and neurodevelopment during the first 5 years of life1. Findings from the COGNIS study showed that the short-term impact of infant formulas and complementary foods enriched with bioactive compounds and human milk-derived probiotics may shape the development of the microbiota-gut-brain axis2,3. Aonghus Lavelle, PhD, from University College Cork, delved into the importance of controlling for confounders (i.e., transit time, regional changes, and horizontal transmission of the microbiome) for improving precision in the field and presented some gut microbiota health (e.g., gut microbiota health index) and disease (e.g., GA-mapTM dysbiosis test) scores. While exploratory analysis (unsupervised microbiome data analysis involving clustering) helps understand disease mechanisms and illuminates targets to modify, microbiome metrics are not ready for clinical application4,5.
The potential of microbiome-based biomarkers for several indications or pathologies. Source: Aonghus Lavelle’s presentation at NeuroGASTRO 2025.
Simone Guglielmetti, PhD, from the University of Milano-Bicocca, explored mechanistic pathways linking microbiome alterations to aging phenotypes and presented recent clinical findings on the benefits of a polyphenol-rich dietary pattern on reducing intestinal permeability and lowering pro-inflammatory gut bacteria-derived mediators. These findings support the fact that it is not the quantity of calories per se that matters but the quality of the diet for healthy aging, even in subjects of advanced age6,7. The bacterial DNA in the blood emerges as a potential microbiome biomarker that may identify vulnerable people who could benefit most from a protective dietary intervention8. Ruairi Robertson, PhD, from Queen Mary University of London, presented recent findings on the influence of the early life microbiome on intestinal development9,10. Unpublished data using a multi-omics approach of a longitudinal cohort study of infants with severe acute malnutrition in Zimbabwe and Zambia showed that a disturbed gut microbiota in early life may lead to increased cysteine/methionine loss and use, contributing to long-term clinical outcomes of malnutrition following treatment.
Mireia Valles-Colomer, PhD, from the University of Pompeu Fabra, presented recent work using two large population cohorts and state-of-the-art bioinformatics tools to strengthen the link between microbial disturbances, depression, and quality of life11. One of the study’s strengths is that the authors mined the literature to identify potential microbial-derived metabolites with neuroactive potential and biochemical pathways, which were subsequently clustered in 56 different gut-brain modules, each corresponding to a single neuroactive compound production or degradation process. Valles-Colomer explained that the next step is to expand this catalogue by adding the so-far uncultivated fraction of the human microbiome and metabolomic analyses to explore the relevance of these gut-brain modules across different neuropsychiatric conditions.

A first catalogue of microbiome neuroactive potential. Source: Mireia Valles-Colomer’s presentation at NeuroGASTRO 2025.
Will the gut microbiome be a new therapy for endometriosis and obesity?
The gut microbiome could also offer hope for managing conditions outside the gut. Siobhain M O’Mahony, PhD, from University College Cork, spoke about direct and indirect mechanisms by which the microbiome influences visceral pain perception and transmission12. A study revealed that 64% of women with endometriosis had Fusobacterium nucleatum infiltration in the uterus. Fusobacterium infection also caused macrophage infiltration, transforming growth factor-b production, and transgelin upregulation in human and mouse endometria as well as endometriotic lesion development in a mouse model of endometriosis13. O’Mahony acknowledged the potential role of microbiome therapy as a novel way for the management of this neuroinflammatory reproductive disorder, as hormones may influence the gut microbiome, which, on the other hand, can also produce sex hormones. Harriet Schellekens, BSc, MSc, PhD, from University College Cork, discussed the successful translational validation of the anti-obesity effects of Bifidobacterium longum APC1472 in otherwise healthy individuals with overweight/obesity14. Unpublished findings in mice also showed that microbiota-targeted interventions with B. longum APC1472 or fructooligosaccharides and galactooligosaccharides can attenuate the enduring effects of early-life high-fat high-sugar, including food intake dysregulation and hypothalamic molecular alterations.

While high-fat, high-sugar treats may be rewarding to the brain, they are not a reward for our gut microbiome. Source: Harriet Schellekens’ presentation at NeuroGASTRO 2025.
‘Gut health’ wellness trend: hype or science?
The claims related to improving, supporting, or maintaining “gut health” are ubiquitous, but researchers have yet to establish a definitive definition. A joint symposium with the European Society of Neurogastroenterology and Motility (ESNM) and the International Scientific Association for Probiotics and Prebiotics (ISAPP) addressed the question of whether gut health is a meaningful concept in neurogastroenterology. Gerard Clarke, PhD, from University College Cork, presented the upcoming ISAPP definition of gut health and acknowledged its main determinants, confounding factors affecting it, and the pros and cons of available biomarkers used to measure gut health in clinical practice. A new ISAPP consensus paper on gut health is forthcoming. Genelle Lunken, PhD, RD, BSc, from the University of British Columbia and the IBD Centre of British Columbia, delved into the dietary nuances in the design, conduct, and reporting of trials to assess gut health. Lunken acknowledged that diet can affect the efficacy of prebiotics and probiotics through changes in the gut microbiome and in the metabolism and expression of genes of the probiotic, thus suggesting that trials of probiotics and prebiotics should consider the impact of background diet as a cofounder. A recent perspective paper in Nature Microbiology summarizes best practices for designing better clinical trials of probiotics and prebiotics.

Defining gut health from fundamental principles to application in clinical practice. Source: Gerard Clarke’s presentation at NeuroGASTRO 2025.
Last, Karen Scott, PhD, from the Rowett Institute of the University of Aberdeen and past ISAPP president, summarized what is new and interesting in ‘biotics’ for gut health. Many studies have assessed the effectiveness of probiotics in gastrointestinal disease, which are outlined in the newly released first edition of the UK Probiotic Guide. Next-generation probiotics are the new kid on the block, with potential applications including metabolic health, reducing gut inflammation in IBD, and increasing satiety, although terminology and claims are not accepted in the EU. Prebiotics are the only ‘biotic’ member for which the European Food Safety Authority has authorized health claims: improving gut health (constipation, gut transit) for inulin and improving glycaemic index for inulin/fructooligosaccharides. Beyond traditional prebiotics, the concept is expanding to include human milk oligosaccharides, resistant starch, polyphenols, dextrose, lactulose, and β-glucan. However, more research is needed to fully characterize these emerging prebiotics and prove that they fit the definition in its entirety. Postbiotics are the newest member of the ‘biotic’ family and offer an opportunity for application of next-generation probiotics that may be hard to deliver through normal routes due to oxygen sensitivity.
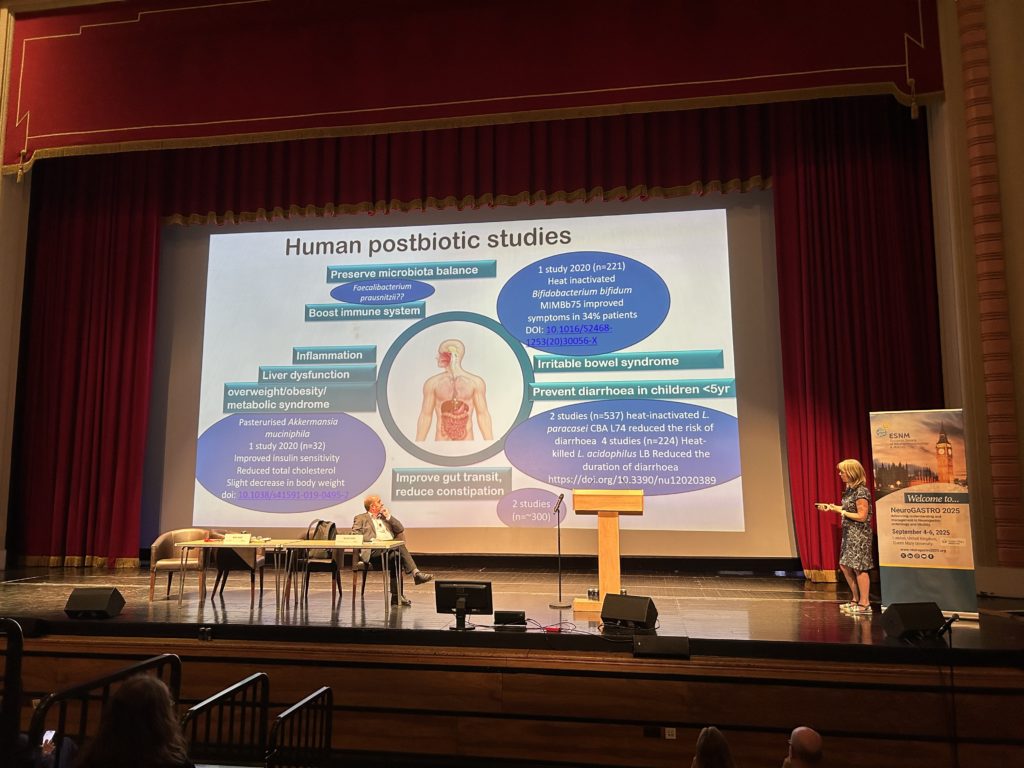
Potential health applications of postbiotics, which are the newest members of the ‘biotics’ family. Source: Karen Scott’s presentation at NeuroGASTRO 2025.
Nutrition matters in gastrointestinal disease
Coverage of the role of nutrition and diet in gastrointestinal disease was strong across the conference. Sarah Berry, PhD, from King’s College London, delivered the keynote talk on the concept of personalized nutrition through the lens of the microbiome. While our gut microbiome is highly variable and unique to each individual (twins share 34% of their gut microbes and unrelated twins share 30% of their gut microbes), it is possible to alter its composition to have a positive impact on health. For instance, if a woman has a gut microbial makeup that enables the conversion of soy isoflavones to equol, then she may experience a 75% greater reduction in some menopause symptoms when supplemented with isoflavones, compared to someone who lacks those specific microbial species15,16.

The future of healthcare through personalized nutrition will involve tailoring health advice to our individual characteristics, dietary habits, and lifestyle. Source: Sarah Berry’s presentation at NeuroGASTRO 2025.
A joint symposium held by the ESNM and the British Dietetic Association featured the first-ever dietary guidelines for the management of chronic primary or idiopathic constipation using a rigorous evidence-based approach (systematic reviews/meta-analyses, GRADE, and Delphi survey) presented by Eirini Dimidi, PhD, RD, from King’s College London. Foods and dietary supplements with effectiveness to improve at least one symptom of chronic constipation include overall fiber supplements, in particular psyllium and inulin-type fructans, some probiotics, specifically multi-strain probiotics, Bifidobacterium lactis, and Bacillus coagulans Unique IS2, magnesium oxide supplements, kiwifruits, prunes, rye bread, and high mineral content water. While a diet high in fiber has been shown to have benefits for gut and overall health, it is not an evidence-based option for constipation. While fermentable fibers have long been contraindicated in patients with upper and lower gastrointestinal disorders, new findings presented by Joshua Reid, PhD, from the University of Nottingham, showed that psyllium delayed colonic fermentation of inulin as assessed by a novel gas-sensing capsule. Reid also presented new findings from the ENERGISE project demonstrating that it is possible to improve tolerance for fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) by utilizing modified fiber gels of methylcellulose and psyllium, which alter the rate of colon gas production differently.
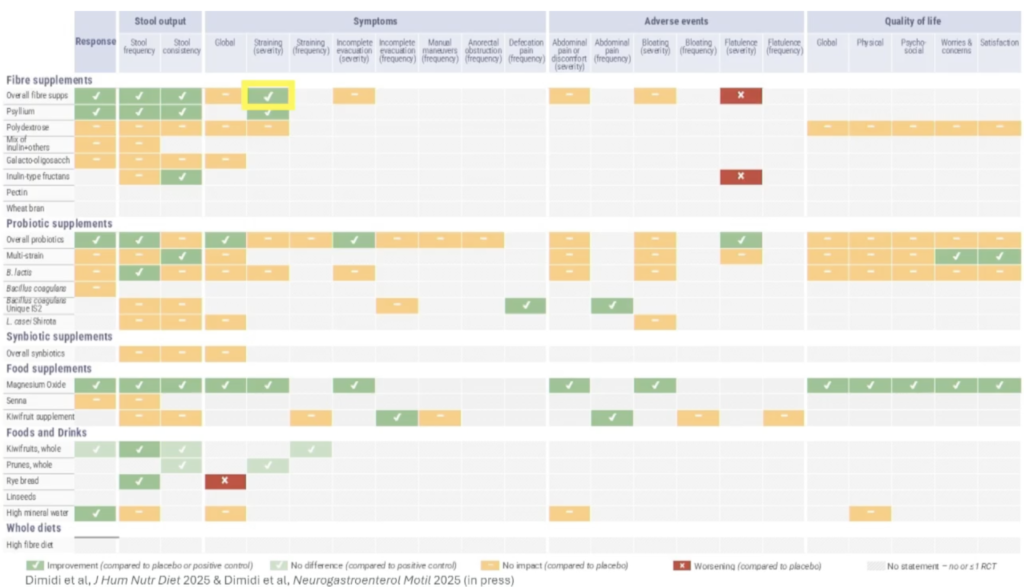
Summary of 59 dietary recommendation statements on dietetic advice for chronic constipation. Source: Eirini Dimidi’s presentation at NeuroGASTRO 2025.
Sanna Nybacka, PhD, RD, from the Chalmers University of Technology and University of Gothenburg, focused on challenges in initiating and adhering to dietary changes in disorders of gut-brain interaction (DGBI). Patients’ challenges to follow the low FODMAP and low-carb diets for IBS include the misalignment between food preferences and beliefs and dietary regimen, difficulties in puzzling the ingredients to whole meals, not knowing where to find reliable sources of information online, and the burden of preparing their own meals17,18.
Mechanistic insights discussed allowed a better understanding of how nutrients shape the gut microenvironment to help develop tailored dietary interventions for DGBI. Mauro D’Amato, PhD, from LUM University, delved into host and microbial genetic factors linked to the efficacy of dietary restriction in DGBI. Genetic variation in sucrase isomaltase (SI) and other human carbohydrate-active enzyme genes may predispose to carbohydrate maldigestion across a continuum of mild to severe bowel symptoms and manifestations, which supports the development of genotype-based dietary interventions. Once considered solely a pediatric condition, SI deficiency may be a mimicker of IBS with diarrhea in adults, especially in individuals who do not respond to the low FODMAP diet and/or other traditional IBS dietary therapies19.
Beyond plenary and short talks, NeuroGASTRO 2025 also included poster sessions, alongside a postgraduate course and a range of awards, including best basic research and clinical articles, ESNM young investigator award, and a lifetime achievement award to Fernando Azpiroz. Other important topics discussed at the conference included the opportunity that digital health strategies provide to help patients with IBS on behalf of the DISCOvERIE project, intestinal and brain barrier dysfunction and the gut-brain axis, new technologies, machine learning and artificial intelligence for the diagnosis and treatment of gastrointestinal motility disorders, and controversies in neurogastroenterology and motility.
The next iteration of NeuroGASTRO will be held in Leuven, Belgium, from 8-10 July 2027.
Further reading:
- Acuña I, Cerdó T, Ruiz A, et al. Infant gut microbiota associated with fine motor skills. Nutrients. 2021; 13(5):1673. doi: 10.3390/nu13051673.
- Cerdó T, Ruíz A, Acuña I, et al. A synbiotics, long chain polyunsaturated fatty acids, and milk fat globule membranes supplemented formula modulates microbiota maturation and neurodevelopment. Clin Nutr. 2022; 41(8):1697-1711. doi: 10.1016/j.clnu.2022.05.013.
- Nieto-Ruiz A, García-Santos JA, Verdejo-Román J, et al. Infant formula supplemented with milk fat globule membrane, long-chain polyunsaturated fatty acids, and synbiotics is associated with neurocognitive function and brain structure of healthy children aged 6 years: the COGNIS study. Front Nutr. 2022; 9:820224. doi: 10.3389/fnut.2022.820224.
- Joos R, Boucher K, Lavelle A, et al. Examining the healthy human microbiome concept. Nat Rev Microbiol. 2025; 23(3):192-205. doi: 10.1038/s41579-024-01107-0.
- Rodriguez J, Hassani Z, Costa Silva CA, et al. State of the art and the future of microbiome-based biomarkers: a multidisciplinary Delphi consensus. Lancet Microbe. 2025; 6(2):100948. doi: 10.1016/j.lanmic.2024.07.011.
- Marino M, Del Bo’ C, Martini D, et al. A (poly)phenol-rich diet reduces serum and faecal calprotectin in older adults with increased intestinal permeability: the MaPLE randomised controlled trial. BMC Geriatr. 2024; 24(1):707. doi: 10.1186/s12877-024-05272-y.
- Del Bo’ C, Bernardi S, Cherubini A, et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: The MaPLE randomised controlled trial. Clin Nutr. 2021; 40(5):3006-3018. doi: 10.1016/j.clnu.2020.12.014.
- Gargari G, Taverniti V, Del Bo’ C, et al. Higher bacterial DNAemia can affect the impact of a polyphenol-rich dietary pattern on biomarkers of intestinal permeability and cardiovascular risk in older subjects. Eur J Nutr. 2022; 61(3):1209-1220. doi: 10.1007/s00394-021-02680-3.
- Robertson RC, Edens TJ, Carr L, et al. The gut microbiome and early-life growth in a population with high prevalence of stunting. Nat Commun. 2023; 14(1):654. doi: 10.1038/s41467-023-36135-6.
- Gough EK, Edens TJ, Carr L, et al. Bifidobacterium longum and microbiome maturation modify a nutrient intervention for stunting in Zimbabwean infants. EBioMedicine. 2024; 108:105362. doi: 10.1016/j.ebiom.2024.105362.
- Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019; 4(4):623-632. doi: 10.1038/s41564-018-0337-x.
- Rea K, O’Mahony SM, Dinan TG, et al. The role of the gastrointestinal microbiota in visceral pain. In: Greenwood-Van Meerveld B (eds). Gastrointestinal Pharmacology. Handbook of Experimental Pharmacology, vol 239. Springer, Cham. doi: 10.1007/164_2016_115.
- Muraoka A, Suzuki M, Hamaguchi T, et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci Transl Med. 2023; 15(700):eadd1531. doi: 10.1126/scitranslmed.add1531.
- Schellekens H, Torres-Fuentes C, van de Wouw M, et al. Bifidobacterium longum counters the effects of obesity: Partial successful translation from rodent to human. EBioMedicine. 2021; 63:103176. doi: 10.1016/j.ebiom.2020.103176.
- Crawford SL, Jackson EA, Churchill L, et al. Impact of dose, frequency of administration, and equol production on efficacy of isoflavones for menopausal hot flashes: a pilot randomized trial. Menopause. 2013; 20(9):936-945. doi: 10.1097/GME.0b013e3182829413.
- Daily JW, Ko BS, Ryuk J, et al. Equol decreases hot flashes in postmenopausal women: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2019; 22(2):127-139. doi: 10.1089/jmf.2018.4265.
- Weznaver C, Nybacka S, Simren M, et al. Patients’ experiences of dietary changes during a structured dietary intervention for irritable bowel syndrome. J Hum Nutr Diet. 2024; 37(5):1336-1348. doi: 10.1111/jhn.13349.
- Trott N, Aziz I, Rej A, et al. How patients with IBS use low FODMAP dietary information provided by general practitioners and gastroenterologists: a qualitative study. Nutrients. 2019; 11(6):1313. doi: 10.3390/nu11061313.
- Zamfir-Taranu A, Löscher BS, Carbone F, et al. Functional variation in human CAZyme genes in relation to the efficacy of a carbohydrate-restricted diet in IBS patients. Clin Gastroenterol Hepatol. 2025; 23(7):1228-1237.e10. doi: 10.1016/j.cgh.2024.09.004.

