A pioneering technology has been developed that enables human kidney organoids to be produced in a scalable manner by allowing the organoids to be combined with ex vivo pig kidneys and then transplanted back into the same animal to evaluate their viability.
The work is published in Nature Biomedical Engineering, in the paper, “Systematic production of human kidney organoids for transplantation in porcine kidneys during ex vivo machine perfusion.” The findings are a significant milestone in regenerative and personalized medicine, paving the way for the use of kidney organoids derived from human stem cells in cell therapy clinical trials.
“Despite the great clinical potential of organoids, one of the major challenges in applying this technology to real medical treatments has been to produce these organoids in a scalable, uniform and affordable way,” says Elena Garreta, PhD, a senior researcher in the IBEC’s Puripotency for Organ Regeneration group. “Now, with our new method, we can generate thousands of kidney organoids under controlled conditions in a short time with great precision, without the need for complex components. This opens the door to applications such as drug screening and disease research.”
The systematic and scalable method for producing thousands of human kidney organoids details a procedure “sustaining the derivation of kidney organoids from hPS cells (hPSC-kidney organoids) using a scalable, reproducible and affordable approach that allows hPSC-kidney organoid differentiation into different renal cell types.”

The team combined human kidney organoids with live pig kidneys connected to normothermic perfusion machines for the first time. These devices are commonly used in operating rooms to keep organs alive and oxygenated outside the body prior to transplantation. They enabled the research team to insert human organoids into pig kidneys and monitor their integration and function in real time.
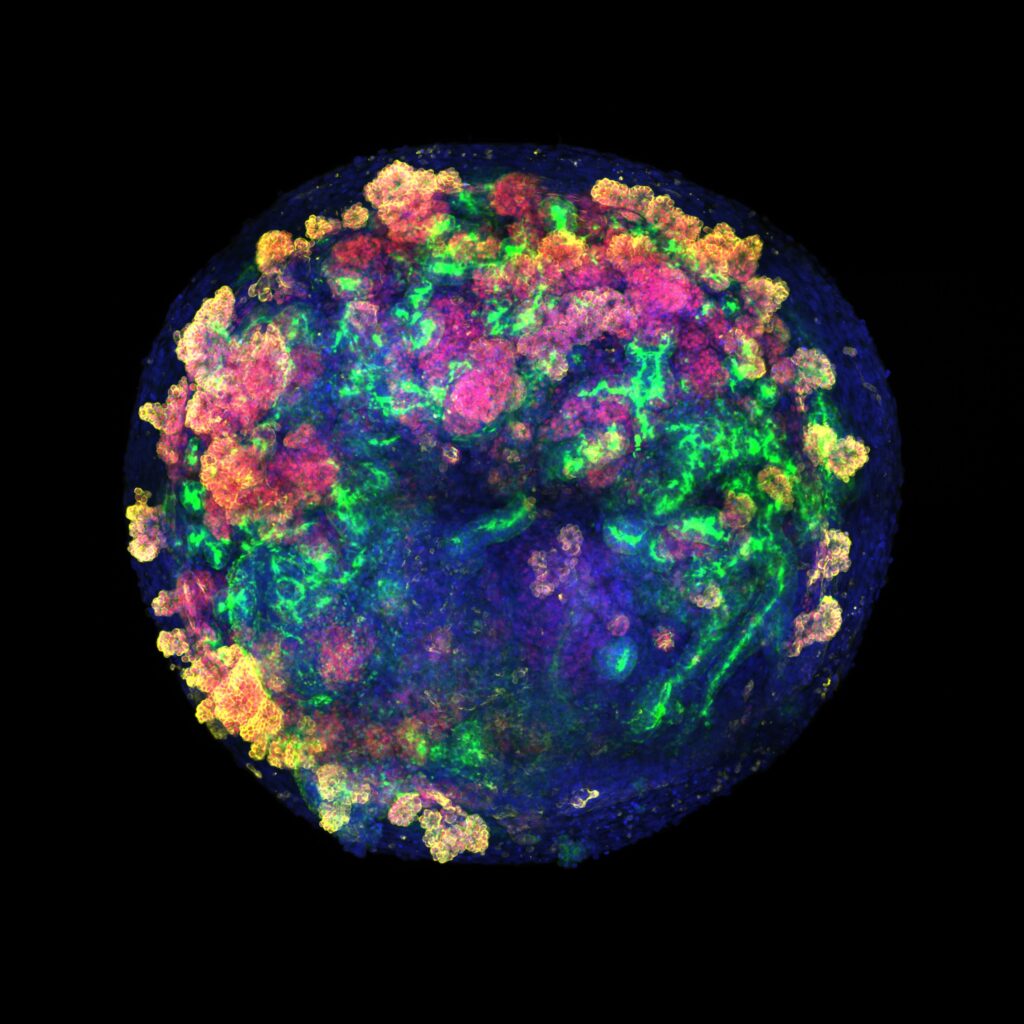
Using single-cell RNA sequencing, confocal image analysis, metabolic assays, and CRISPR–Cas9 engineering for generation of fluorescent reporters, they show that hPSC-kidney organoids “exhibit transcriptional variety and cellular composition following cell-to-cell contact.”
“Our research shows that combining organoid and ex vivo perfusion technologies can enable cellular interventions under fully controlled conditions,” explains Núria Montserrat, PhD, a principal investigator at Institute for Bioengineering of Catalonia (IBEC) at the time of the study and now Minister of Research and Universities of the Government of Catalonia. “The long-term goal is to be able to regenerate or repair an organ before transplantation. This could reduce waiting times for chronic patients and increase the number of viable organs for transplantation.”
Perfusing the organoids within the kidneys using the aforementioned machines offers a key advantage: it allows the physiological parameters of the organ to be measured in real time, enabling any signs of damage or rejection to be detected immediately.
In addition to performing ex vivo experiments, the team also used a porcine transplant model that is highly similar to the human kidney for in vivo experiments. They observed that, 24 and 48 hours after transplantation, the human organoids remained integrated into the porcine renal tissue. More specifically, they further evaluated the immune response, “confirming the feasibility and viability of the procedure. We identify cells of human origin after normothermic machine perfusion and in vivo transplantation.”
The transplanted kidney continued to function normally and there were no signs of damage or toxicity. According to the authors, this methodology enables us to envisage a clinical scenario in which organs intended for transplantation can be treated and prepared prior to implantation.