All cells, from bacteria to mammalian cells, produce extracellular vesicles—tiny membrane-enclosed packages of bioactive cargo.1 Until relatively recently, scientists believed these organic nanoparticles were just useless cellular debris, but with newer data, researchers now recognize them as crucial components of intercellular signaling.
“[Extracellular vesicles are] like the text message system of the body, whether that’s long distances, so from one organ to another, like stomach to the brain, or short distances between cells in the same tissue,” said Joy Wolfram, a nanoscientist at the University of Queensland. Found in all human biofluids, these nano-sized fat bubbles are filled with important cargo, including proteins, nucleic acids, and lipids, through which they elicit a range of physiological and pathological effects.2 Wolfram explained that while extracellular vesicles are complex and heterogeneous, they can be sorted into three broad categories. “There’s the good ones or the therapeutic ones, there’s the neutral ones that don’t really have either a beneficial or bad effect, and then there’s the ones that drive disease,” she said.
Bad Extracellular Vesicles Drive Metastasis of Triple-Negative Breast Cancer
A cryogenic electron microscopy image of extracellular vesicles, resembling a smiley face, within the holes of a perforated support film. The background was artificially shaded light gray to highlight the vesicles.
Wolfram Laboratory
Bad extracellular vesicles are one of Wolfram and her group’s primary focus areas, particularly in the context of triple-negative breast cancer (TNBC). According to Wolfram, when TNBC becomes aggressive and metastasizes to other parts of the body, it implies a failure of the immune system: the cancer cells become immune evasive, either by shutting down immune cells or otherwise hiding from them.
“Our data suggests that it’s the extracellular vesicles that help this process in terms of hiding from the immune system, but what we’re also seeing is that they’re actually suppressing the function of the immune system, and specifically the natural killer cells, which are the first line of defense against cancer,” she remarked.
Their research revealed multiple mechanisms by which extracellular vesicles negatively modulate the tumor microenvironment in TNBC, making it more immune-suppressive or less accessible to immune cells.3 Direct interactions, in which bad EVs bind to the surface of natural killer cells, can cause the cells to be ‘switched off’. Indirect interactions, which are less common, involve the extracellular vesicles being internalized by the natural killer cells and suppressing their function.
In a recent study published in the Journal of Extracellular Vesicles, Wolfram and her team found that the EVs secreted by TNBC can actually ‘hitchhike’ on bad cholesterol molecules, revealing another possible mechanism of tumor growth and metastasis that could lead to better treatments.4
Anti-Inflammatory Extracellular Vesicles Reduce Open-Heart Surgery Complications

Joy Wolfram and her team at The University of Queensland study extracellular vesicles in the context of triple-negative breast cancer, cardiovascular disease, and more.
Alex Druce, The University of Queensland
In contrast, Wolfram and her team also study good extracellular vesicles and hope to apply them as therapeutics to reduce harmful inflammation in the context of cardiovascular disease and open-heart surgery. “When they put the patient on the surgery, all the inflammatory cytokines go up. They stay up for a long time,” Wolfram commented. “30 percent of the patients have complications, even higher than that, maybe 10 percent need [treatment in the intensive care unit], and a small percentage actually die from that inflammatory response that actually affects the whole body.”
In collaboration with John Fraser, an intensive care specialist at the Prince Charles Hospital, Wolfram is using anti-inflammatory extracellular vesicles from mesenchymal stem cells to reduce the inflammation caused by surgery, providing better clinical outcomes for patients.
In addition to natural EVs isolated from human biofluids, Wolfram and her colleagues also aim to engineer them to enhance their therapeutic properties. While delivering therapeutic compounds can be achieved through other methods, Wolfram said that extracellular vesicles provide a unique advantage: “We can engineer them, for example, to carry mRNA that then codes for a protein that is anti-inflammatory, such as proteins that need to be membrane-bound to have an effect.”
Isolation And Standardization Challenges in Extracellular Vesicle Research
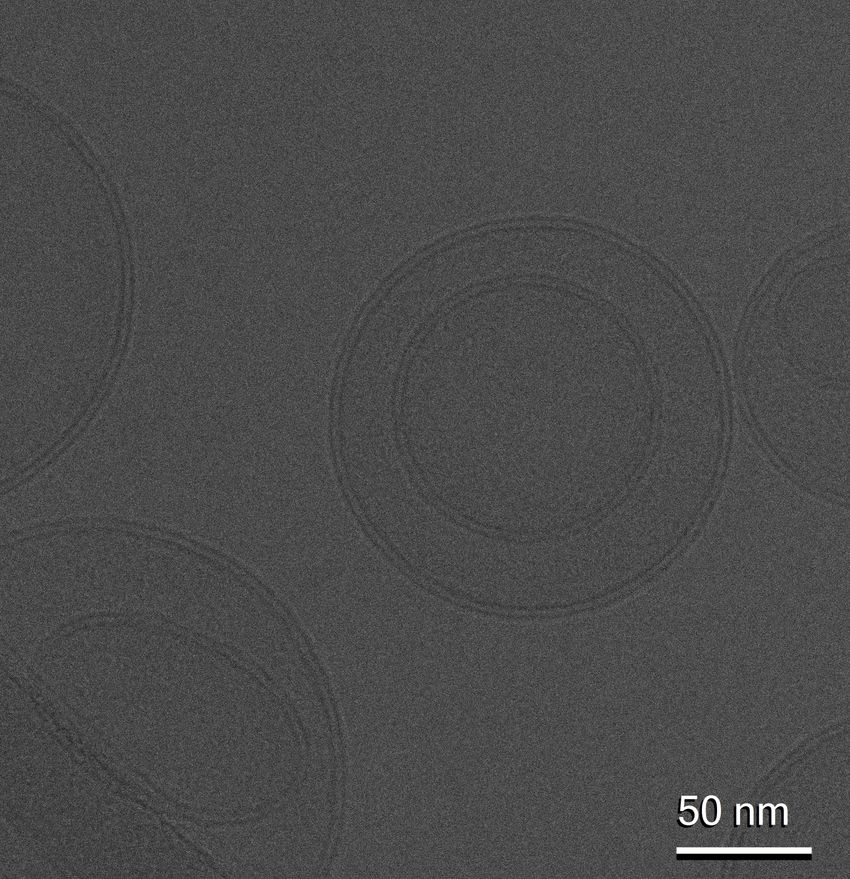
One of the key obstacles to the use of extracellular vesicles as therapeutics is that isolation techniques, such as ultracentrifugation, can damage them.
Wolfram Laboratory
Despite its promise, extracellular vesicle research is not without its challenges, and like many burgeoning research fields, there is a lack of standardization and consistency. Currently, Wolfram said, most laboratories use ultracentrifugation as the primary isolation method, which damages the tiny fat bubbles. “Then [researchers] are saying, ‘when we inject them intravenously, they all go to the liver,’. Yes, but they’ve also been damaged, because it’s like, you in a roller coaster times a thousand,” she remarked.
Another cause of the lackluster results of extracellular vesicle clinical trials is incorrect storage; the vast majority of EV studies and clinical trials do not use a cryoprotectant when freezing the therapeutic materials to -80°C. “You would never freeze a cell without a cryoprotectant. You would never freeze a virus without a cryoprotectant, you would never freeze an LNP, without a cryoprotectant,” Wolfram observed. “…One of our previous papers showed that when you use cryoprotectant, you have less damage and you have more surface preservation.”
Isolating sufficient numbers of natural extracellular vesicles, Wolfram said, is also a major challenge globally because it requires “football fields of cells” grown in culture. “One of the things we’re trying to do is overcome the cell culture needs, and instead use, for example, human blood as a library of every single extracellular vesicle you could imagine, because every cell in the body is in very close contact with the blood,” Wolfram noted.
However, therein lies another significant challenge. Extracellular vesicles are incredibly heterogeneous, so how can researchers sort through an enormous library of them and categorize them all? “Even if you have the exact same cell, in the exact same environment, it will still release different types [of EVs], because there are EVs that are essentially made in different ways, but then if you put it in a bit more stress, the cell is more stressed, or the cell is in a slightly different environment, those EVs will change as well,” Wolfram explained. “So just an estimate [of how many types of EVs there are] is around the number of cells times 10,000 if not more.”
Overcoming regulatory hurdles for extracellular vesicle therapeutics. Because of the extreme complexity and heterogeneity of extracellular vesicles, they also face regulatory and clinical challenges. This has led Wolfram to investigate the development of mimetics that use clinically approved nanoparticles loaded with components of good extracellular vesicles, like proteins. “Then we see things like improved endosomal escape, which is a huge bottleneck, especially for mRNA,” Wolfram said.
Wolfram and her team have already collaborated with several companies that currently have therapeutics in clinical trials, but she said they hope to develop more home-grown extracellular vesicle therapeutics in the coming years. “Our goal is to get to those early-phase clinical trials and then to partner with industry to enable us to save lives,” she added.
Frequently Asked Questions
What are extracellular vesicles?
- Extracellular vesicles are nano-sized, lipid-bound vesicles that carry important bioactive cargo, such as nucleic acids, proteins, and lipids.5 Secreted by every cell type in the body, these tiny molecular packages allow cells to communicate over long and short distances in the body, similar to a text message sent between cells.
Different types of extracellular vesicles
- EVs can be divided into three major categories based on their formation: exosomes, microvesicles, and apoptotic bodies.6 However, because of their extreme heterogeneity in size, biogenesis, and physiological functions, the classification of extracellular vesicles is still debated by researchers.
- Recent research suggests that EVs can be further classified into more diverse subtypes, including ectosomes, oncosomes, and microparticles.6 Scientists like Wolfram often classify EVs as good, bad, or neutral based on their effects in the body.
Extracellular vesicles vs. exosomes: What’s the difference?
- Exosomes are a subtype of extracellular vesicle. The difference between exosomes and other EVs is primarily based on their biogenesis: exosomes are formed when the endosome (a membrane-bound cell organelle) buds inward and later fuses with the lipid bilayer of the cell, allowing the resulting exosome to be secreted.7
Extracellular vesicle formation
- The three primary categories of EVs are based on their biogenesis. As we discussed above, exosomes are formed by an inward budding of the endosome and later merge with the cell’s plasma membrane. In contrast, ectosomes are generated by an outward budding of the cell’s plasma membrane. Finally, apoptotic EVs are fragments of an apoptotic (dying) cell.7
Extracellular vesicle size
- Extracellular vesicles are extremely heterogeneous in terms of their size, ranging between 30nm and 10µm in diameter.8
- Pendiuk Goncalves J, et al. High-throughput analysis of glycan sorting into extracellular vesicles. Biochim Biophys Acta BBA – Mol Cell Res. 2024;1871(2):119641.
- Kumar MA, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9(1):27.
- Coleborn E, et al. Cancer-derived extracellular vesicles in natural killer cell immune evasion: Molecular mechanisms and therapeutic insights. Mol Ther. 2025;0(0).
- Ghebosu R, et al. Lipoprotein association fluorometry (LAF) as a semi-quantitative characterization tool to assess extracellular vesicle-lipoprotein binding. J Extracell Vesicles. 2025;14(10):e70172.
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727.
- Teng F, Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv Sci. 2021;8(1):2003505.
- Jeppesen DK, et al. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023;33(8):667-681.
- Johnson SM, et al. Large extracellular vesicles can be characterised by multiplex labelling using imaging flow cytometry. Int J Mol Sci. 2020;21(22):8723.