Cell lines
B16-F0 (ATCC; CRL-6322) and its LN metastatic derivatives: NBF0-LN1-18IL, NBF0-LN7-1112AR, NBF0-LN7-1120BL, NBF0-LN7-1134BL, NBF0-LN8-1194BR, NBF0-LN8-1198AR, NBF0-LN8-1205BL, NBF0-LN9-1315BL and NBF0-LN9-1358IR—were provided by the Reticker-Flynn Laboratory. For simplicity, these cell lines are referred to throughout the manuscript as: B16-F0, LN1-18IL, LN7-1112AR, LN7-1120BL, LN7-1134BL, LN8-1194BR, LN8-1198AR, LN8-1205BL, LN9-1315BL and LN9-1358IR, respectively. B16F10 wild-type (WT), B16F10 Fsp1-KO and B16F10 Gpx4-KO cells were obtained from the Conrad Laboratory. B16-F0 Fsp1-KO, LN7-1134BL Fsp1-KO, LN9-1315BL Fsp1-KO, B16-F0 Gclc-overexpression, LN7-1134BL Gclc-overexpression, B16-F0 Gclc-KO and B16-F0 Nrf2-overexpression lines were generated in this study. Human melanoma cell lines MeWo, SK-MEL-5, A375, murine melanoma lines Yale University Melanoma Model (YUMM) 3.3 and YUMM 5.2, and HEK293T cells were purchased from ATCC. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, 11885076) supplemented with 10% FBS (Thermo Fisher Scientific, 26400044) and 1% penicillin–streptomycin (Thermo Fisher Scientific, 15140122). All of the other lines were authenticated by ATCC using STR profiling. Cells were routinely tested for mycoplasma contamination using MycoStrip (InvivoGen, rep-mys-50).
Chemicals
RSL3 (HY-100218A), erastin-2 (HY-139087), iFSP1 (HY-136057), BTZ (HY-10227) and PEG300 (HY-Y0873) were purchased from MedChemExpress. ML-210 (S0788), MG-132 (S2619) and icFSP1 (E1535) were acquired from Selleck Chemicals. Rotenone (R8875), oligomycin (75351), antimycin A (A8674), L-BSO (B2515), N-acetyl cysteine (A9165), Na2SeO3 (S5261), CQ (C6628) and PEG400 (202398) were obtained from Sigma-Aldrich. FCCP (15218), MTT (21795), GSHee (14953), liproxstatin-1 (17730), IMP-1088 (25366), NSC 624206 (20569), FSEN1 (38025), viFSP1 (39927) and triacsin C (10007448) were obtained from Cayman Chemical Company. MitoView Fix 640 (70082) and LipidSpot 488 (70065) were sourced from Biotium. Lipofectamine 3000 (L3000015), Bodipy 581/591 C11 (D3861), SYTOX Green (S7020), Lysotracker Deep Red (L12492) and NucBlue Live ReadyProbes Reagent (R37605) were from Thermo Fisher Scientific.
Plasmids
pCMV3-FSP1-OFP plasmid (MG52065-ACR) was obtained from Sino Biological. Lenti-luciferase-P2A-neo (Addgene, 105621), psPAX2 (Addgene, 12260), pMD2.G (Addgene, 12259) and PX458 (Addgene, 48138) were obtained from Addgene. Custom constructs including pTWIST-mFSP1-G2A-OFP, pLVX-EF1α-GCLC-IRES-Hygro, and pLVX-EF1α-NRF2-IRES-Hygro were synthesized by Twist Bioscience and cloned into expression vectors using Gibson Assembly.
Generation of stable cell lines
Stable cell lines expressing luciferase, GCLC or NRF2 were generated through lentiviral transduction followed by antibiotic selection. Lentivirus was produced by co-transfecting HEK293T cells with 5 µg of either Lenti-luciferase-P2A-neo, pLVX-EF1α-GCLC-IRES-Hygro or pLVX-EF1α-NRF2-IRES-Hygro, combined with 5 µg psPAX2 and 0.5 µg pMD2.G using Lipofectamine 3000. Virus-containing supernatants were collected every 24 h for 48 h, filtered and supplemented with 8 µg ml−1 Polybrene (Sigma-Aldrich, H9268). Target cells were infected and subsequently selected with either 1,500 µg ml−1 G418 or 1,000 µg ml−1 hygromycin B for 6 days to establish stable populations.
CRISPR–Cas9-mediated gene KO
To generate Fsp1– or Gclc-KO cell lines in B16-F0 and its LN metastatic derivatives, sgRNAs were designed with BbsI-compatible overhangs and cloned into the PX458 Cas9-GFP vector. The sgRNA sequences were as follows: Fsp1 (CACCGGCGGCTGCCAGCCAGCTGC) and Gclc (CACCGGGGAGTTACATGATCGA). sgRNA insertion was confirmed by whole-plasmid sequencing. Cells were transfected with PX458-sgRNA constructs using Lipofectamine 3000 and GFP-positive cells were sorted by flow cytometry and expanded. Transfection and cell sorting was repeated a second time to generate a pure population for expansion prior to validation. KOs were validated by western blotting and Sanger sequencing (Extended Data Fig. 8i,j for FSP1 and Extended Data Fig. 5j for GCLC).
LN9-1315BL Fsp1-KO cell lines were generated by lentiviral transduction using the LCv2_Blast vector containing mouse Fsp1 sgRNA 1 (sequence: CACCGCCGTGCACGTGGTGATCGT), previously validated43. Transduced cells were selected with 5 µg ml−1 blasticidin. KO validation is shown in Extended Data Fig. 9c.
Western blot analysis
Cell lysates (15–20 μg protein) were separated by SDS–PAGE, transferred onto PVDF membranes (Bio-Rad, 1620177), blocked with 5% non-fat milk in TBS-T or PBS-T, and incubated with primary antibodies overnight at 4 °C in 5% non-fat milk in PBS-T. After washes, the membranes were incubated with HRP-conjugated secondary antibodies and proteins detected by enhanced chemiluminescence (Thermo Fisher Scientific, 32106). The following antibodies were used: ACSL3 (Abcam, ab151959, 1056272-1, WB,1:5,000, Ms), ACSL4 (Santa Cruz Biotechnology, A-5, I1222, WB,1:200, Ms), actin (MP Biomedical, 691001, 0101008716, WB, 1:20,000, Ms and Hu), FSP1 (Proteintech, 20886-1-AP, 00111298, WB,1:2,000, KD validated in-house, Ms and Hu), anti-mouse IgG HRP (Cell Signaling, 7076S, 36, WB, 1:5,000), anti-rabbit IgG HRP (Cell Signaling, 7074S, 33, WB, 1:5,000), COX IV (Cell Signaling, 4850, 11, WB, 1:1000, Ms), GAPDH (Santa Cruz Biotechnology, 6C5, J2523, WB, 1:20,000, Ms), GCLC (Santa Cruz Biotechnology, H-5, J0621, WB, 1:2,000, KO validated in-house), GPX4 (Abcam, ab125066, lot 1000287-43, WB, 1:2,000, KO validated in-house), HIF-1α (Cell Signaling, 36169, 5, WB, 1:1,000), LAMP1 (Abcam, ab24170, GR3235630-1, WB, 1:1,000, Ms), LAMP2A (Abcam, ab18528, 1029399-1, WB, 1:1,000, Ms), LC3 (Cell Signaling, 3868, 14, WB, 1:1,000), LIMPII (Proteintech, 27102-1-AP, WB), NRF2 (Proteintech, 16396-1-AP, 00116728, WB, 1:5,000), NRF2 (Proteintech, 80593-1-RR, 23013625, WB, 1:1,000), PDIA3 (AMAB90988, WB, 1:200), RCAS1 (Cell Signaling, 12290S, D2B6N, 6, WB, 1:1,000), SCL7a11/xCT (Cell Signaling, 98051, 1, WB, 1:300), ubiquitin (Cell Signaling, 43124T, 4, WB, 1:1,000), γ-tubulin (Cell Signaling, T5326, WB, 1:1,000).
Immunoprecipitation and ubiquitination detection
B16-F0 and LN7 1134BL cells were incubated under normoxic (21% O2) or hypoxic conditions (1% O2) for 16 h. Proteins were extracted with RIPA buffer plus protease and phosphatase inhibitors. For denatured immunoprecipitation, lysates were heated to 95 °C for 5 min. Both native and denatured lysates were incubated with anti-GPX4 antibody (Proteintech, 67763-1-Ig, 10027815) or mouse IgG control (Proteintech, B900620) overnight at 4 °C, followed by incubation with anti-mouse IgG Sepharose beads (Cell Signaling, 5946) for 6 h at 4 °C. Beads were washed with RIPA buffer and analysed by immunoblotting using the anti-ubiquitin antibodies (Cell Signaling, 43124T, 4, WB, 1:1,000).
IHC analysis
A TMA containing primary cutaneous melanoma and LN metastases (ME551; TissueArray.com) was used to assess the expression of GCLC, GPX4 and FSP1. The sections were stained with antibodies against GPX4 (Abcam, ab125066, 1:500), GCLC (Santa Cruz, sc-390811, 1:500) and FSP1 (Proteintech, 68049-1-Ig, 1:500) using the Zytomed Permanent AP Red Kit (ZUC001-125) according to the manufacturer’s instructions, followed by counterstaining with haematoxylin. The slides were scanned with an Axio Scan.Z1 slide scanner (Zeiss). Quantification of AP Red signal intensity was performed using QuPath (v.0.5) with uniform thresholding parameters across all samples.
FSP1 enzyme activity
NADH consumption assays were performed in PBS (Gibco, 14190094) containing 15 or 25 nM recombinant non-myristoylated human FSP1, 100 μM menadione (Sigma-Aldrich, M5625) and 200 μM NADH43. The pH of the final reaction was adjusted from 4.0 to 9.0 by titrating PBS with HCl or NaOH. After the addition of FSP1, the absorbance at 340 nm was recorded every 20 s at 37 °C using the SpectraMax M5 microplate reader (Molecular Devices). Reactions lacking NADH or enzyme were included for background correction. Data were normalized and fitted using GraphPad Prism 10.
Confocal fluorescence microscopy
Cells plated on coverslips were transfected with FSP1-OFP using Lipofectamine 3000. After 16 h, cells were treated with IMP-1088 (0.1 μM) for 24 h. Cells were fixed (4% paraformaldehyde), permeabilized (0.1% Triton X-100), and incubated overnight with primary antibodies in 3% BSA/PBS and then with by Alexa-Fluor-conjugated secondary antibodies. For live-cell imaging, cells were plated on 30-mm glass-bottom dishes, transfected as described above, and incubated with Lysotracker (50 nM) and NucBlue Live ReadyProbes reagent during the final 30 min of IMP-1088 treatment. Images were captured with a Nikon Eclipse Ti confocal microscope using consistent settings for comparisons and analysed with Fiji software. Antibodies and stains used included Alexa Fluor 647 donkey anti-rat (Thermo Fisher Scientific, A48272, YK388772, IF, 1:500), Alexa Fluor 488 goat anti-rabbit (Thermo Fisher Scientific, A32731, YI374177, IF, 1:500), Alexa Fluor 546 goat anti-rabbit (Thermo Fisher Scientific, A11010, 2570547, IF, 1:500), ERp72 (Cell Signaling, 5033, 4, IF, 1:200) GPX4 (Abcam, ab125066, 1000287-7, IF, 1:100, KO validated in-house) from Abcam; LAMP1 (Thermo Fisher Scientific, 14-1071-82, 2698949, IF, 1:50), RCAS1 (Cell Signaling, 12290, 6, IF, 1:200), MitoView Fix640 (70082-50 μg, 23M0201-1215003) and LipidSpot 488 (70065, 22L0820) from Biotium.
Lipid oxidation assays
Cells (60,000 per well) were seeded in 12-well plates one day before treatment. Cells were treated with 0.5 µM RSL3 for 4 h or 1% O2 for 24 h, washed with PBS, trypsinized and resuspended in PBS containing 1.5 µM C11-BODIPY 581/591 (Invitrogen, D3861). After 30 min incubation at 37 °C, cells were washed, incubated with DAPI, filtered through a 70-µm strainer and analysed on the BD LSR Fortessa flow cytometer. Excitation was performed at 488 nm, detecting oxidized BODIPY (FITC, 525/40 nm) and reduced BODIPY (PE, 585/42 nm). At least 10,000 events were analysed per sample. Data were processed using FlowJo software, and the lipid oxidation ratio (FITC/PE ratio) was calculated as (median FITC-A − median FITC-A unstained)/(median PE-A − median PE-A unstained). The flow cytometry gating strategies for the lipid oxidation assays are presented in Supplementary Fig. 2.
Cell viability and cell death assays
Cells (2,500–3,000 per well) were seeded into 96-well plates. Viability was measured using MTT assay 24 h (erastin-2) or 48 h (RSL3, ML-210, viFSP1 + BSO and Triacsin C) after treatment. Cell death was monitored every 3 h using SYTOX Green (25 nM) in the Incucyte S3 (Sartorius) system.
Isolation of lysosome-enriched fractions
Lysosome-enriched fractions were isolated using the Lysosome Isolation Kit (Abcam, ab234047) according to the manufacturer’s protocol. In brief, 2 × 107 cells were washed and centrifuged at 600g for 10 min and the supernatant was removed. Cells were resuspended in Lysosome Isolation Buffer, vortexed and incubated on ice for 2 min. Complete cell disruption was obtained using a dounce homogenizer. After adding Lysosome Enrichment Buffer, the homogenate was centrifuged at 500g for 10 min at 4 °C. The supernatant was added to the top of a discontinuous gradient density and an ultracentrifugation at 145,000g for 2 h at 4 °C was performed. The lysosome-enriched fraction was present in the top 10% of the gradient volume. For western blot analyses, the protein content of the lysosomal-enriched gradient supernatant was quantified using the Qbit 1 fluorometer (Thermo Fisher Scientific) and a protein quantification kit (Thermo Fisher Scientific, Q33212). Equal total protein amounts of total cell extracts and lysosome-enriched extracts were loaded for comparison for western blot analyses.
Isolation of Golgi-enriched fractions
Golgi-enriched fractions were isolated using the Golgi enrichment extraction kit (Invent, GO-037) according to the manufacturer’s instructions. In brief, filter cartridges were placed and cooled on ice for several minutes. Then, 2 × 107 cells were trypsinized and collected by centrifugation at 500g, washed with 1× PBS and centrifuged again at 500g. The pellet was resuspended in buffer A with vigorous shaking. The filter cartridge was capped, the tube inverted several times and centrifuged at 16,000g for 30 s. The tube was then centrifuged at 4 °C at 5,000g for 5 min without removing the filter. The filter was then removed and the supernatant transferred to a fresh tube and centrifuged at 4 °C at 16,000g for 30 min. The supernatant was then transferred to a fresh tube. An equivalent in volume of buffer B was added to the supernatant, the resulting mixture incubated on ice for 15 min and then centrifuged at 8,000g for 5 min. The pellet was then resuspended in buffer A and mixed by pipetting up and down 50 times and subsequently centrifuged at 8,000g for 5 min. The supernatant was then transferred to a fresh tube and ice old buffer C was added, mixed by vortexing for 20 s and incubated on ice for 20 min. The tube was then centrifuged at 8,000g for 10 min and the supernatant removed. The pellet was resuspended Laemmli buffer for subsequent western blot analysis. For western blot analyses, the protein content of the Golgi-enriched extracts was quantified using the Qbit 1 fluorometer (Thermo Fisher Scientific) and a protein quantification kit (Thermo Fisher Scientific, Q33212). Equal total protein amounts of total cell extracts and lysosome-enriched extracts were loaded for comparison for western blot analyses.
Isolation of ER-enriched fraction
ER were isolated using the ER enrichment extraction kit (Novus Biologicals, NBP2-29482) according to the manufacturer’s instructions. In brief, 500 µl of 1 × isosmotic homogenization buffer followed by 5 µl of 100× PIC were added to a pellet of 2 × 107 cells. The resulting suspension was centrifuged at 1,000g for 10 min at 4 °C. The supernatant was transferred to a clean centrifuge tube and centrifuged at 12,000g for 15 min at 4 °C. The floating lipid layer was discarded. The supernatant was centrifuged in a clean centrifuge tube using an ultracentrifuge at 90,000g for 1 h. The resulting pellet contained the total ER fraction (rough and smooth). The pellet was resuspended Laemmli buffer for subsequent western blot analysis. For western blot analyses, the protein content of the ER-enriched extracts was quantified using the Qbit 1 fluorometer (Thermo Fisher Scientific) and a protein quantification kit (thermo Fisher Scientific, Q33212). Equal total protein amounts of total cell extracts and lysosome-enriched extracts were loaded for comparison for western blot analyses.
Mitochondrial/cytoplasmic fractionation
Mitochondrial and cytoplasmic fractions were obtained using a mitochondria isolation kit for mammalian cells (89874) from Thermo Fisher Scientific according to the manufacturer’s instructions.
RNA-seq analyses
RNA-seq data were generated and analysed as described previously15. Raw sequencing reads were trimmed and quality-filtered using Trimmomatic and FastQC, respectively. Transcript abundance was quantified with Salmon v.0.7.2 using quasi-mapping mode and corrected for sequence, GC and positional biases, using the mouse genome GRCm38 GENCODE release M11. TPM values were computed using tximport and renormalized after removing mitochondrial transcripts. Differential expression analysis was performed using DESeq2 with regularized log-transformed counts. Hierarchical clustering and PCA analyses used Spearman correlations from the top 1,000 highly variable genes. Heat maps (Extended Data Fig. 1a) were generated using heatmap3 from the top 200 differentially expressed genes. Data have been deposited in the Gene Expression Omnibus (GEO: GSE117529).
ATAC–seq analyses
ATAC–seq analyses were conducted as described previously15. In brief, cells were permeabilized and DNA was transposed using Tn5 transposase. Libraries were purified, amplified and sequenced (NovaSeq, 2 × 100 cycles, around 50 million paired reads per sample). Reads were mapped to mm10 (hisat2), duplicates removed (Picard) and peaks were called using MACS2. Normalized coverage was visualized in IGV. Transcription factor activity and motif enrichment were assessed with Chromvar and HOMER, respectively. Data were deposited at the GEO (GSE117529).
RNA isolation and qPCR analyses
RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, 74134), and cDNA was synthesized using the iScript Reverse Transcription Supermix (Bio-Rad, 1708841). qPCR was performed using the iTaq Universal SYBR Green Supermix (Bio-Rad, 1725121) on the BioRad CFX96 system. The primers used were as follows: mNRF2_F: AACGACAGAAACCTCCATCTAC; mNRF2_R: AGTAAGGCTTTCCATCCTCATC; mFSP1_F: GCAATGAGTATCGGGAGTACAT; mFSP1_R: GTAGGCAGAGCTGTTGATCTT; mGPX4_F: ACTGACGTAAACTACACTCAGC; mGPX4_R: GGAAGGCCAGGATTCGTAAA; RNA pol II_F: ACTGTGCGGAACTCCATCAA; RNA pol II_R: AGCCAGGTTCTGGAACTCAA; mPPIB_F: CATCAAGGACTTCATGATCCA; mPPIB_R: ATAGATGCTCTTTCCTCCTGTG. RNA pol II and PPIB amplification were used as reference genes. PPIB was used as a housekeeping gene for qPCR analyses of parental and LN metastatic lines, while RNA Pol II was used for qPCR analyses of BTZ treatment under 21% and 1% O2 conditions.
Metabolite extraction and LC–MS analysis
For metabolite extraction, 5 × 105 cells were seeded into 6-well plates and cultured for 24 h. The medium was then aspirated, and cells were washed with cold normal saline (9 g l−1 sodium chloride). Immediately, 400 µl of extraction buffer (methanol:acetonitrile:water, 40:40:20, with 0.5% formic acid) was added per well, and the plates were incubated on ice for 5–10 min. The samples were neutralized with 35 µl of 15% ammonium bicarbonate (NH4HCO3), cells were scraped and lysates were transferred to 1.5 ml tubes and centrifuged at 16,000 rpm for 15 min. A total of 80 µl of supernatant was transferred to LC–MS vials, and 20 µl from each sample was pooled to generate a quality control sample. All of the extracts were stored at –80 °C until analysis.
Metabolites were analysed using a Q Exactive HF mass spectrometer (Thermo Fisher Scientific) coupled to hydrophilic interaction chromatography (HILIC). Separation was performed using an XBridge BEH Amide XP column (2.5 µm, 2.1 × 150 mm) with a guard column (2.5 µm, 2.1 × 5 mm; Waters). Mobile phase A consisted of water:acetonitrile (95:5) and mobile phase B comprised water:acetonitrile (20:80), both containing 10 mM ammonium acetate and 10 mM ammonium hydroxide. The gradient was as follows: 0–3 min, 100% B; 3.2–6.2 min, 90% B; 6.5–10.5 min, 80% B; 10.7–13.5 min, 70% B; 13.7–16 min, 45% B; 16.5–22 min, 100% B. The flow rate was 0.3 ml min−1. The autosampler was maintained at 4 °C and the column at 30 °C. The injection volume was 5 µl. Needle washes were performed between injections using acetonitrile:methanol:water (4:4:2, v/v/v).
MS1 scans were acquired from m/z 70 to 1,000 with polarity switching and a resolution of 120,000 (at m/z 200). Other MS parameters were as follows: sheath gas, 40; auxiliary gas, 10; sweep gas, 2; spray voltage, 3.5 kV; capillary temperature, 300 °C; S-lens RF level, 45; maximum injection time, 500 ms; AGC target, 3 × 106.
Raw data were converted to mzXML format using msConvert and analysed in El-Maven (Elucidata) for targeted metabolite identification based on accurate mass and retention time, using an in-house standard library. Data were normalized to protein content and analysed in MetaboAnalyst 6.0 (https://www.metaboanalyst.ca).
GSH measurements
Cells (5,000 per well) were seeded into 96-well plates, and GSH levels were assessed using the GSH/GSSG-Glo assay (Promega, V6611). Parallel cell viability assessments were used for data normalization.
Seahorse assay
Cells (5,000 per well) were seeded in 96-well plates and analysed using the Seahorse XF24 system. Oxygen consumption rates were measured sequentially after oligomycin (1 μM), FCCP (1 μM) and rotenone/antimycin A (0.5 μM each). Data were normalized to protein content.
s.c. and i.n. tumour models
Mice were housed under sterile conditions with sterilized standard chow and water provided ad libitum and maintained under a 12 h–12 h light–dark cycle and 22 ± 2 °C, 55 ± 5% humidity. Animals were allocated randomly to treatment groups, and the samples were processed in an arbitrary order. No formal randomization or blinding was applied. The maximum permitted tumour diameter of 2.0 cm was not exceeded in any of the experiments. All procedures complied with institutional ethical guidelines and were approved by the Institutional Animal Care and Use Committee of the Harvard T.H. Chan School of Public Health (protocol IS00003460) or the Stanford University Institutional Animal Care and Use Committee (protocol APLAC-34518).
For s.c. injections, 2 × 105 B16-F10 WT Luc, B16-F10 Fsp1-KO Luc, or LN7 1134BL WT or Fsp1-KO cells were suspended in 100 µl of DMEM without phenol red and injected into either the right or left flank of 6–8-week-old male or female C57BL/6J or C57BL/6N mice44.
For i.n. injections, 1 × 104 SK-MEL5 or LN7 1134BL WT or Fsp1-KO cells were injected into the popliteal LN of 6–8-week-old NSG or C57BL/6J mice. To visualize the lymphatics, 2% Evans Blue dye (Sigma-Aldrich, E2129) was injected into the footpad 5 min before the procedure. Mice were injected with buprenorphine and anesthetized with isoflurane, and a 5–10 mm incision was made in the region of the right popliteal LN. The node was identified by Evans Blue staining, immobilized with forceps and 1 × 104 cells in 10 µl of 1× PBS were injected into the LN using a 27 G Hamilton syringe. Successful injection was confirmed by visible swelling of the node. Incisions were closed with surgical glue (VetBond Tissue Adhesive, 3M, 1469SB) and the mice were monitored for signs of pain or distress for 5 days45.
Once tumours were palpable in ≥50% of mice (around 1 week after injection), 10 µl of vehicle or drug solution was administered daily through intratumoural (i.n. or s.c.) injection into tumour-bearing sites. Treatment groups included: L-BSO (1 mM; Thermo Fisher Scientific, 235520050), icFSP1 (0.025 mg (2.5 mg ml−1); Selleckchem, E1535), L-BSO + icFSP1 (1 mM + 0.025 mg (2.5 mg ml−1)), viFSP1 (0.025 mg (2.5 mg ml−1); MedChemExpress, HY-163002), L-BSO + viFSP1 (1 mM + 0.025 mg (2.5 mg ml−1)) and FSEN1 (0.025 mg (2.5 mg ml−1); MedChemExpress, HY-153629). L-BSO was dissolved in 0.9% sodium chloride (saline; Quality Biology, 114-055-101). icFSP1 was formulated in 55% PBS (Corning, VWR45000-430) and 45% PEG300 (MedChemExpress, HY-Y0873). viFSP1 and FSEN1 were formulated in 20% DMA, 40% PEG400 and 40% of 50% 2-hydroxypropyl-β-cyclodextrin (2HPβCD) in water.
Tumour diameters were measured daily using callipers until any tumour reached around 1.5 cm in its largest dimension, which defined the experimental end point. At the end point, all of the mice in the cohort were euthanized in accordance with approved protocols. Tumour diameters and weights were recorded, and tissues were collected and frozen for downstream analyses.
Experimental lung metastasis was evaluated through intravenous delivery of cancer cells in the lateral tail vein of tumour-naive mice. A total of 2 × 106 LN7-1134BL WT or Fsp1-KO cells was resuspended in 200 µl of DMEM without phenol red and injected into the lateral tail vein of 8-week-old female C57BL/6N mice using a 27-gauge needle44. Mice were euthanized 14 days after injection, and the lungs were inflated with PBS using a 25-gauge needle inserted into the trachea, and the lungs were removed for visible counting of metastatic nodules identified by melanin.
For LN spontaneous metastasis assays, 2 × 105 LN7 1134BL WT or Fsp1-KO cells were suspended in 100 µl DMEM (without phenol red) and injected s.c. into the right or left flank of 6–8-week-old male or female C57BL/6J or C57BL/6N mice. Mice were euthanized 24 days after injection and the draining LNs were collected and classified as metastatic (LN+) or non-metastatic (LN−) based on the presence of melanin-containing melanoma cells44.
Bioinformatics analysis
Correlation analyses used tools available online (https://hgserver1.amc.nl/). Metabolomic data were analysed using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/).
Joint pathway analysis transcriptomic and metabolomic datasets showing significant alterations (P < 0.05, |Fold Change| > 1) between parental (B16-F0) and LN (LN8) clones underwent joint pathway enrichment analysis using MetaboAnalyst. Parameters included integrated metabolic pathways, hypergeometric test, degree centrality topology and pathway-level P-value combination. Pathways were considered significant at P < 0.05 and impact > 0.2 (normalized degree centrality), with at least two significantly altered metabolites.
Correlation analysis gene–metabolite correlations were calculated using the cor.test function (R stats package v.3.6.2). Analysis focused on highly interconnected genes and metabolites within the KEGG glutathione metabolism pathway modules (glutathione biosynthesis and ferroptosis protection), obtained using the MetaboSignal package (v.1.32.1) and the cluster_walktrap algorithm from the igraph package (v.2.0.2). Only late LN tumour generations were included due to sample size limitations.
Bayesian inference of directed acyclic graphs (DAGs) was used to identify cause–effect networks among genes and metabolites across tumour generations (early: B16-F0, F018IL; late: LN7, LN8, LN9). DAG networks were inferred using the BiDAG package (v.2.1.4) with Bayesian Gaussian equivalent scoring and order Markov Chain Monte Carlo structure learning. Networks were averaged over 100 iterations to account for inference variability, assigning edge probabilities based on inference frequency.
Software for Illustrations
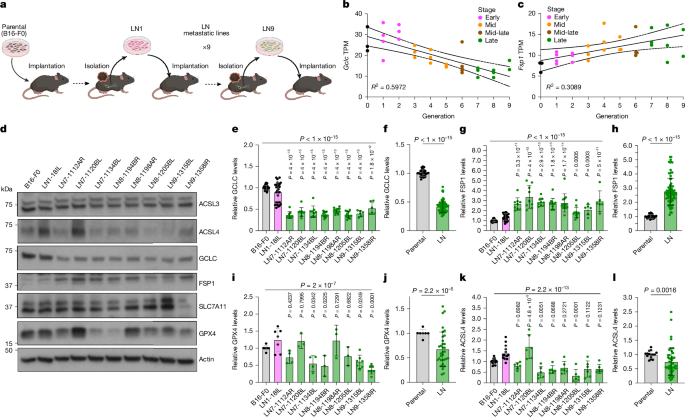
Illustrations were generated using FIJI (2.0.0-rc-69/1.52n), Prism (10.5.0) and BioRender (http://biorender.com). Figures created using BioRender include Figs. 1a, 2c and 5j and Extended Data Figs. 7i and 10a,b.
Statistical analysis
Data are presented as mean ± s.d. Statistical analyses were performed using GraphPad Prism v.10.5.0 (GraphPad Software) and included unpaired two-sided Student’s t-tests with Welch’s correction, one-way ANOVA with Dunnett’s, Tukey’s or Šidák’s multiple-comparisons tests, Kruskal–Wallis tests followed by Dunn’s post hoc test, log-rank (Mantel–Cox) tests for survival analyses and contingency analysis using χ2 with Fisher’s exact test. P < 0.05 was considered to be statistically significant. Sample sizes (n) refer to biological or technical replicates as defined in individual figure legends. Numbers independent biological replications are indicated in the figure legends, with the exception of Fig. 1, for which replicates are noted here: for Fig 1e,g, B16-F0 (n = 30), LN1-18IL (n = 30), LN7-1112AR (n = 9), LN7-1120BL (n = 9), LN7-1134BL (n = 9), LN8-1194BR (n = 12), LN8-1198AR (n = 12), LN8-1205BL (n = 12), LN9-1315BL (n = 6), LN9-1358IR (n = 6); Fig. 1f,h, parental (n = 30), LN (n = 75); Fig. 1i, B16-F0 (n = 7), LN1-18IL (n = 7), LN7-1112AR (n = 4), LN7-1120BL (n = 3), LN7-1134BL (n = 4), LN8-1194BR (n = 3), LN8-1198AR (n = 3), LN8-1205BL (n = 3), LN9-1315BL (n = 7), LN9-1358IR (n = 7); Fig. 1j, parental (n = 7), LN (n = 34); (k) B16-F0 (n = 15), LN1-18IL (n = 15), LN7-1112AR (n = 6), LN7-1120BL (n = 6), LN7-1134BL (n = 6), LN8-1194BR (n = 6), LN8-1198AR (n = 6), LN8-1205BL (n = 6), LN9-1315BL (n = 6), LN9-1358IR (n = 6); Fig. 1l, parental (n = 15), LN (n = 48).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.