In 2011, a devastating earthquake struck the eastern part of Japan, triggering the Fukushima nuclear power plant accident and resulting in widespread radioactive contamination, including in agricultural fields. In the following years, Tatsuya Nobori worked in a plant physiology lab as a undergraduate and master’s student in Tomoko Nakanishi’s group at the University of Tokyo, where he focused on understanding how plants interacted with these radioactive substances.
The lab’s work examined how rice—one of Japan’s staple crops—absorbed radioactive cesium, particularly Cesium-137, a major contaminant in the affected areas. Nobori was deeply involved in both laboratory work and fieldwork, conducting surveys and investigating how these plants uptake and accumulate nutrients and Cesium-137.1
Although he did not initially have strong aspirations to become a scientist, he explained that through his fieldwork studies, “I [became] quite interested in the ecological aspect, because I realized that everything is connected.” What struck him most was the broader ecological fallout—how radioactive material contaminated tree leaves, which then entered the soil, get absorbed by mushrooms, and eventually consumed by animals or people. “We surveyed the circulation of [these] elements in the ecosystem.”
During this project and through an internship in genetics and molecular biology, Nobori became captivated by plant biology, recognizing that this fundamental research can have real-world impact. This sparked his passion for the field and has guided his work ever since, leading him to investigate plant-microbe interactions and plant immunity.
I was particularly interested in the heterogeneity within plant microbe interactions and how individual cells of plants and microbes might be interacting.
—Tatsuya Nobori, The Sainsbury Laboratory
From Visualizing Plant Genes with PHYTOMap to Creating an Arabidopsis Atlas
Plants, fixed in the soil, face constant stress from harsh weather, such as drought, as well as microbial infections that enter through wounds or natural openings in the plant or are transmitted by insect vectors. To survive, plants have evolved a powerful innate immune system to fend off microbial pathogens.2
Yet, despite extensive research, how plant immunity ultimately suppresses bacterial growth remains unclear, largely because it is difficult to track bacterial responses within the plant. Intrigued by this, Nobori dove into several method development projects to create in planta bacterial transcriptomics and proteomics technologies during his doctoral studies at the Max Planck Institute for Plant Breeding Research.
Many of his projects focused on tissue-level studies investigating how the leaves and microbes within the leaves would interact, though not necessarily in the individual cells.3 However, towards the end of his doctoral research, he remarked, “I was particularly interested in the heterogeneity within plant-microbe interactions and how individual cells of plants and microbes might be interacting.”
Then, around 2019, new single-cell omics and spatial-omics technologies emerged, primarily in the biomedical field, but had not yet taken off in plant research. So, Nobori thought it would be interesting to apply these methods to studying plants. As he searched for a postdoctoral position, he joined plant biologist and molecular biologist Joseph Ecker’s group at the Salk Institute for Biological Studies. There, Ecker’s team worked on both plant and animal models and had used single-cell omics techniques on the animal side.
“I kind of naively thought that by using single-cell sequencing, we could understand how individual cells respond and communicate,” Nobori explained. At the time, the available omics methods were based on cutting very thin tissue sections to capture plants interacting with microbes; however, these only visualized genes in a 2D space. “But then I quickly realized that that’s also not enough to really understand how plant and microbial cells communicate because those interactions happen in 3D space, and by cutting tissue slices, you’re going to lose a lot of critical information,” he said.
As Nobori tested several spatial analysis methods for plants, Ecker shared a protocol from Stanford University neuroscientist Karl Deisseroth. Deisseroth’s STARmap (Spatially-resolved Transcript Amplicon Readout Mapping) allowed for 3D mapping of gene expression within intact mouse brain tissue.4
Nobori developed PHYTOMap, a spatial gene expression mapping technique, that allows researchers to visualize gene activity across individual cells in 3D.
Salk Institute
“Tatsuya said, ‘Wow, that’s exactly what we need to do.’…He was ready to hit the ground running,” Ecker remarked. Nobori started working on a protocol for a plant system, particularly the model plant Arabidopsis thaliana, to look at gene expression in 3D.
As Nobori refined his methods, he found conversations with biophysicist and postdoctoral fellow Robert Henley, who was developing other spatial omics technologies in mouse brains, particularly insightful. Henley, who was also in Ecker’s group, taught Nobori many fundamental techniques and theoretical concepts. Drawing on these exchanges, Nobori combined STARmap with hybridization-based in situ sequencing (HybISS) schemes which culminated into what became known as a plant hybridization-based targeted observation of gene expression map (PHYTOMap).5,6
Built upon in situ hybridization and sequencing technologies, PHYTOMap simultaneously visualized dozens of genes within major cell types and developmental stages within the 3D whole-mount plant tissues. As a proof-of-concept, he mapped the root tip, a critical structure for investigating growth, nutrient acquisition, and interactions with soil microbes in A. thaliana.
“He’s the kind of person that has tenacity and will just go after it,” said Ecker.
Nobori kept busy with numerous projects: incorporating single-cell multiomics technologies and spatial transcriptomics, such as multiplexed error-robust fluorescence in situ hybridization (MERFISH), and building a transcriptome atlas of A. thaliana across 10 developmental stages. This atlas, in collaboration with fellow Salk Institute postdoctoral researchers Travis Lee and Natanella Illouz-Eliaz, spanned 10 developmental stages. Nobori described this as “seed-to-seed:” from seedlings to mature plant leaves, flowers, and next-generation seeds.7 “We’re very excited about this work,” he said and added that this project originated from Ecker, who “wanted us to do something big and useful for the plant science community.”
For this project, the team sequenced nearly 800,000 cells in the initial data set. Then, with new spatial transcriptome methods, they targeted hundreds of genes from each type of tissue to create a map of gene expression, cell types, and cellular states within tissues from different developmental stages of plants. Nobori remarked that this atlas’s data is publicly available online so scientists all over the world can browse through their favorite genes and check whether those genes are expressed in certain tissues at different times of development.
While the atlas represents normal development of A. thaliana, Nobori would like to build on this atlas to include information on how plants respond to different stresses and environmental conditions. In addition, he believes that this atlas could serve as a valuable reference for researchers to predict gene functions in the context of different organs and cell types, thereby guiding efforts to engineer plants at the cellular level.
Illuminating the Defenses Plants Use Against Microbial Attack
As Nobori developed methods and references, his main project focused on plant immunity. Because plants don’t know when or where the next pathogen attack will occur, plant cells are masters of multitasking. However, researchers really didn’t know how individual plants would respond to this random and unpredictable pathogen attack.
To tackle this mystery at a more granular level, Nobori wanted to know more: Which cells respond first, and how do they influence one another when activating immune responses at the levels of gene expression and epigenetic regulation?
So, he infected A. thaliana leaves with bacterial pathogens that trigger or suppress immune responses. Then, armed with his repertoire of various transcriptomic technologies, he could characterize how specific cells across the leaf activate the plant’s immune responses.
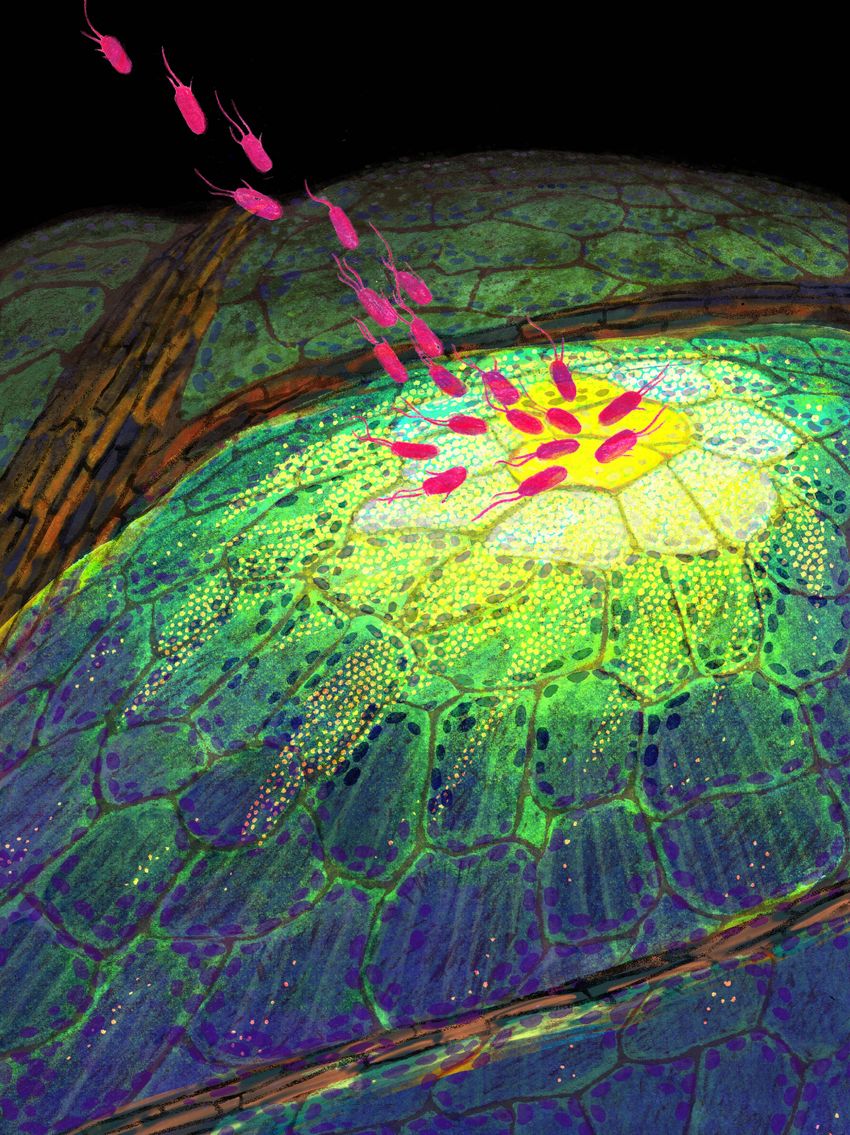
Using a spatial transcriptomics method (MERFISH), Nobori revealed gene expression in the leaf of Arabidopsis thaliana infected by a bacterial pathogen.
Hsuan Pai
Nobori identified a small group of cells, which he called primary immune responder (PRIMER) cells, that have a different profile than neighboring cells, which he called the bystander cells.8 “The PRIMER cell is like a smoke detector, and the bystander cells might be like a sprinkler,” he explained. “This primary cell detects the pathogens. But attacking the pathogen might be done by the bystander cell, so there may be some division of labor to make more efficient defenses.”
As these PRIMER cells signal to bystander cells during a pathogen attack, Nobori remarked that the next layer of complexity is to understand the real function of these cells, which remains unclear, and how these cellular immune states are changing temporally. Although it’s early days in determining whether this process is a general mechanism shared among other plants, he hopes to apply a similar method to study many different plant pathogen interactions. As new experimental and computational technologies are introduced, Nobori is on the lookout for how he can incorporate these methods to address his biological questions.
Ecker added that molecular geneticist Ronald Davis, a research fellow during Ecker’s own time as a postdoctoral researcher at Stanford University, would often say, “You should spend 50 percent of your time developing new tools and the other 50 percent studying biology [with that tool].” That way, researchers can become pioneers of what they’ve developed on the technological side and then apply it to biology. “Tatsuya came perfectly into that mold. He’s continuing to push the technology to be able to get better information from the spatial aspect and then doing cutting-edge biology with that,” Ecker said.
Planting the Seeds of Discovery: Nobori’s New Lab Takes Root
In 2023, Nobori set his sights on new horizons—this time, across the Atlantic, at The Sainsbury Laboratory (TSL). His work caught the attention of Thorsten Langner, then a plant pathologist at TSL, who first encountered Nobori’s work through his application for a group leader position.
“One of the things that immediately struck me was the quality and clarity of [Nobori’s] presentation,” said Langner, referring to Nobori’s work on MERFISH and multiomics approaches. “It’s really enjoyable to see his talks.”
Nobori officially joined TSL in 2024. Since then, he and Langner—now based at the Max Planck Institute for Biology Tübingen—have continued to cross paths at conferences and scientific gatherings. Most recently, they reconnected as invited speakers at the 14th Conference of the European Foundation of Plant Pathology, hosted in Sweden in the summer of 2025.
“Tatsuya is one of these scientists who is fun to talk science with,” Langner said. “You can see that he’s really into it, and that he really kind of lives for it.”
During a conversation at the conference, the two exchanged ideas about Nobori’s recent research on how plants respond to pathogen attacks at the single-cell level. Langner found the work compelling and wondered aloud about its broader applications in more complex tissues and across different species.
From his conversations with Nobori, Langner remarked, “He’s a very collaborative person, so he’s always looking for new collaborations. He has a very, very strong technology at hand that he’s now trying to spread a little bit in the community. He’s always looking for people that work on different systems to help to establish this method on a larger scale.”
I think [Nobori’s] ahead of the curve…He has a vision that he wants to contribute to shaping plant science and particularly plant pathology.
—Joseph Ecker, The Salk Institute for Biological Studies
Reflecting on his early work in plant biology, Nobori remarked, “It made me realize that it kind of circles back to my initial ecological interest and how single-cell level interactions might have a ripple effect on the actual ecosystem. It is definitely a very important perspective to have before [researchers] deploy any of that engineering to the field.”
Now, his main goal is to gain a deep understanding of the cellular building blocks of the plant immune system, such as the kinds of cellular states that emerge during immune activation, their functions, regulation pathways, and overall interactions with one another.
Looking ahead, Nobori’s next goal is to advance the PHYTOMap technology to eventually map target microbes along with plant genes and visualize how they might interact with each other. He added that the next phase of work is determining how to harness this knowledge to engineer or optimize plant-microbe interactions in nature and agricultural setups for a more sustainable agricultural ecosystem. By gaining a better understanding of plant-microbe interactions, researchers can more effectively target cells or cell states to manipulate or engineer their responses for beneficial traits such as better yield, disease resistance, or nutrient efficiency without posing much risk for side effects.
“I think [Nobori’s] ahead of the curve…He has a vision that he wants to contribute to shaping plant science and particularly plant pathology,” said Ecker.

