In a proof of concept that may reshape the treatment landscape for insulin-dependent diabetes, scientists have demonstrated that human stomach cells can be reprogrammed to secrete insulin—potentially paving the way for autologous cell-based therapies that eliminate the need for donor islets and systemic immunosuppression, as well as lifelong monitoring of blood sugar levels and insulin injections.
The study, “Modeling in vivo induction of gastric insulin-secreting cells using transplanted human stomach organoids,” published in Stem Cell Reports and led by Xiaofeng Huang, PhD, of Weill Cornell Medicine and Qing Xia, MD, PhD, of Peking University, shows that human gastric tissue can be transformed in vivo into functional insulin-producing cells using a precision combination of defined genetic factors. The work builds on earlier findings in mice that the stomach’s cellular architecture can be coaxed into producing insulin and represents the first demonstration that this conversion can occur in human-derived tissues inside a living organism.
Type 1 diabetes (T1D), which affects an estimated 9.5 million people worldwide, results from autoimmune destruction of pancreatic beta cells—the body’s only natural source of insulin. Current treatments depend on lifelong insulin administration or experimental stem cell-derived islet transplants, both of which have limitations, including immune rejection and limited donor availability. “Functional insulin-secreting cells can be induced in situ from the murine stomach using defined genetic factors, offering a promising method to directly produce autologous insulin-secreting cells. Here, we modeled whether such gastric insulin-secreting (GINS) cells could be generated in vivo from human stomach tissues,” the authors wrote.
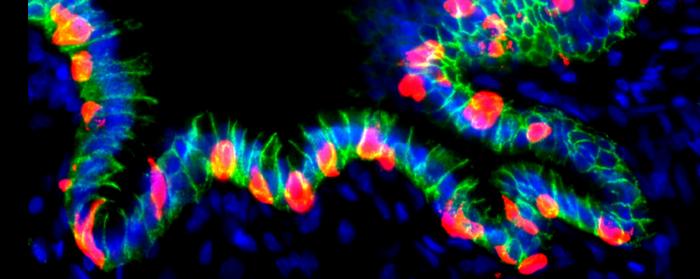
To test whether this approach could extend to humans, the researchers engineered human gastric organoids (hGOs)—three-dimensional “mini-stomachs” derived from human embryonic stem cells. They inserted an inducible cassette encoding three key pancreatic reprogramming factors—NEUROG3, PDX1, and MAFA—collectively termed NPM. These factors were integrated at the AAVS1 safe-harbor locus to allow doxycycline-controlled activation without disrupting essential genes.
When transplanted into immunodeficient mice, the hGOs survived for up to six months and matured, developing complex epithelial and mesenchymal structures reminiscent of native gastric tissue. Upon activation of the NPM “genetic switch,” human stomach cells within these graphs began expressing insulin and key beta-cell markers, including PCSK1, NKX2-2, PAX6, and MAFB—a transcription factor uniquely present in human, but not murine, beta cells.
Functionally, these engineered GINS cells released insulin into the bloodstream and significantly improved blood glucose control in diabetic mice. In animals treated with doxycycline to trigger NPM expression, blood glucose levels rapidly normalized and remained stable, while control mice showed a partial, slower recovery. “Compared with the control group, which received no Dox, there was a rapid amelioration of blood glucose in the Dox-treated animals, which was maintained until the end of the experiment at approximately six weeks,” the authors wrote. Human insulin was detected in the serum of treated mice, confirming active secretion by the transplant organoids.
“These data suggest the feasibility of inducing GINS cells in situ in the human stomach,” the authors concluded. The approach could, in theory, allow physicians to reprogram a patient’s own gut mucosa to produce insulin directly within the body—bypassing donor shortages and reducing the risks associated with systemic immunosuppression.
Still, the researchers caution that extensive safety and efficacy testing remains ahead. The study used a single embryonic stem cell line, and the induced cells did not yet organize into islet-like structures. Another limitation was that long-term hyperglycemia was not maintained at a steady level in the transplanted mice, which showed partial recovery. But as proof of concept, the findings mark a major step toward harnessing the regenerative potential of human tissues to restore insulin production—an approach that could one day offer a functional cure for type 1 diabetes.