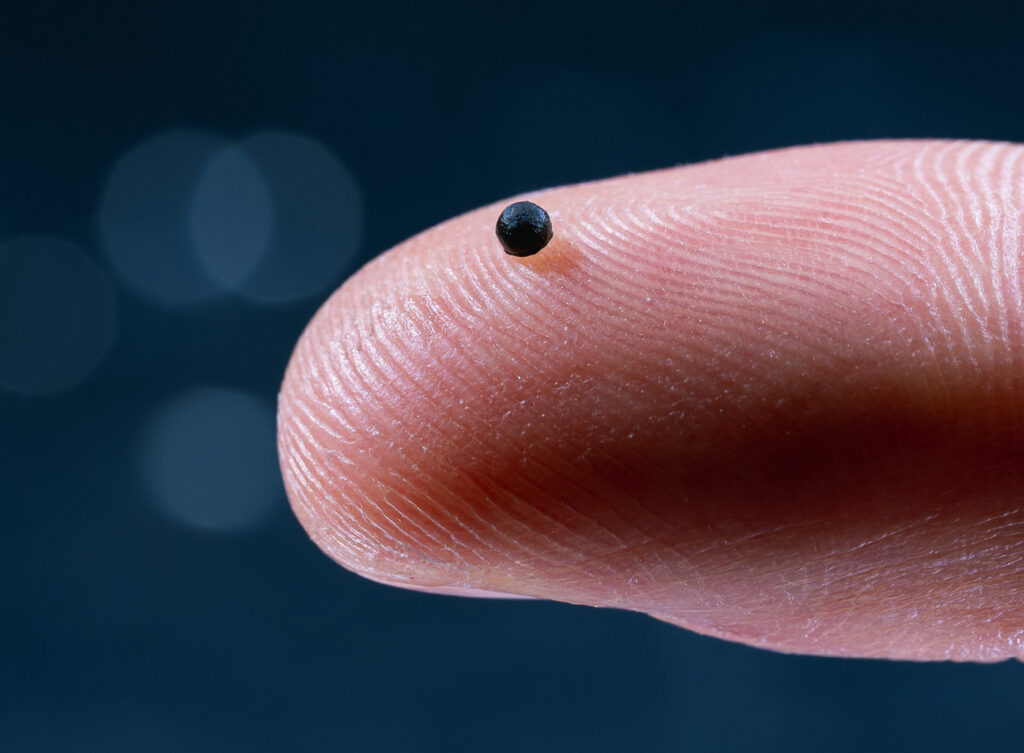
Researchers headed by a team at ETH Zurich have developed a magnetically guided microrobotics system that they say can navigate the body’s intricate passageways and vasculature to deliver drugs with pinpoint accuracy. Headed by Fabian Landers, PhD, a postdoctoral researcher at the Multi-Scale Robotics Lab at ETH Zurich, the team’s modular, magnetically guided microrobotic platform integrates an electromagnetic navigation system (Navion) with a custom release catheter and drug-loaded, dissolvable capsule.
The investigators tested the platform in vitro using human vasculature models and in vivo in sheep and pigs under realistic clinical conditions to demonstrate its capabilities. Their tests demonstrated that by applying specific magnetic fields, the system can be made to maneuver through complex blood vessels and cerebrospinal spaces, trigger controlled heating to dissolve the microrobot, and precisely release drugs into targeted tissues, even reaching the smallest vessels.
The team said their novel system could enable safer, targeted drug treatments that minimize unwanted side effects. Lead author Landers and colleagues described the platform in Science, in a paper titled “Clinically ready magnetic microrobots for targeted therapies.” In their report, the team concluded, “Our findings demonstrate the ability to navigate microrobots in vivo within large animal models under clinical conditions by integrating navigation, therapeutic delivery, and imaging into a unified platform.”
Every year, 12 million people worldwide suffer a stroke, and many die or are permanently impaired. Treatment involves administering drugs to dissolve the thrombus that blocks the blood vessel. These drugs spread throughout the entire body, meaning that a high dose must be administered to ensure that the necessary amount reaches the thrombus. Systemic delivery can cause serious side effects, such as internal bleeding.
“Severe side effects are often associated with systemic administration of drug treatments and are responsible for 30% of drug failures during clinical trials,” the authors further wrote. Since medicines are often only needed in specific areas of the body, medical research has long been searching for a way to use microrobots to more precisely deliver pharmaceuticals to targeted sites: in the case of a stroke, directly to the stroke-related thrombus.
Advances in materials science, fabrication, and control systems have enabled microrobots capable of complex movement and targeted delivery in complex biological environments. “… magnetic micro- and nanorobots have been the focus of extensive research over the past two decades,” the researchers commented. “These devices hold tremendous potential for targeted drug delivery, offering the possibility of delivering higher concentrations of therapeutic agents directly to disease sites, thereby enhancing treatment efficacy and minimizing side effects.” However, bridging these technologies into clinical practice remains challenging.
Building on their previous work, Landers and colleagues have now developed a microrobot that comprises a proprietary spherical capsule made of a soluble gel shell that they can control with magnets and guide through the body to its destination. Iron oxide nanoparticles in the capsule provide the magnetic properties. “Because the vessels in the human brain are so small, there is a limit to how big the capsule can be. The technical challenge is to ensure that a capsule this small also has sufficient magnetic properties,” explained Lander.
The microrobot also needs a contrast agent to enable doctors to track via X-ray how it is moving through the vessels. Describing the system in summary, the team wrote, “The magnetic microrobot consists of a spherical gelatin matrix embedded with highly responsive magnetic iron oxide nanoparticles, radiopaque tantalum nanoparticles, and a therapeutic agent, materials that have been previously FDA approved for various intravascular applications.”
The researchers focused on tantalum nanoparticles, which are commonly used in medicine but are more challenging to control due to their greater density and weight. “Combining magnetic functionality, imaging visibility, and precise control in a single microrobot required perfect synergy between materials science and robotics engineering, which has taken us many years to successfully achieve,” explained co-senior author and ETH Professor Bradley Nelson, PhD, who has been researching microrobots for decades. Co-senior author Professor Salvador Pané, PhD, a chemist at the Institute of Robotics and Intelligent Systems, and his team developed precision iron oxide nanoparticles that enabled this delicate balancing act.
The microrobots contain the active ingredient they need to deliver. The researchers successfully loaded the microrobots with common drugs—a thrombus-dissolving agent, an antibiotic, and tumor medication—for a variety of applications. The drugs are released using a high-frequency magnetic field that heats the magnetic nanoparticles, dissolving the gel shell and the microrobot.
The researchers used a two-step strategy to bring the microrobot close to its target. The microbot is first injected into the blood or cerebrospinal fluid via a catheter. “The design uses a commercial catheter (7 Fr) with an inner guidewire connected to a flexible polymer gripper,” they commented. “When pushed beyond the outer guiding catheter, the polymer gripper opens and releases the preloaded microrobot.”
![Graphical representation of the various navigation options. [ETH Zurich]](https://www.genengnews.com/wp-content/uploads/2025/11/low-res-3-300x169.jpeg)
The electromagnetic navigation system (eMNS), Navion, is then used to precisely steer and guide the magnetic microrobot to the target location. The catheter’s design is based on a commercially available model with an internal guidewire connected to a flexible polymer gripper. When pushed beyond the external guide, the polymer gripper opens and releases the microrobot.
The modular eMNS system is suitable for use in the operating theater. In their report, the team explained, “We coupled two Navion systems, each featuring three electromagnetic coils separated by a distance of 35 cm, to facilitate magnetic manipulation within a calibrated workspace large enough to accommodate a human skull.”
The researchers combined three different magnetic navigation strategies, which allowed them to navigate in all regions of the arteries of the head. “Using rotating magnetic field–based rolling, magnetic gradient–based pulling, or in-flow navigation, our untethered microrobot achieves multimodal navigation,” the authors stated. “The speed of blood flow in the human arterial system varies a lot depending on location,” Nelson added. “This makes navigating a microrobot very complex.”
The platform allows the investigators to roll the capsule along the vessel wall using a rotating magnetic field. The capsule can be guided to its target with enormous precision at a speed of 4 mm per second. In a different model, the capsule is moved using a magnetic field gradient: the magnetic field is stronger in one place than in another. This pulls the microrobot in the vessel towards the stronger field. The capsule can even go against the current, and at a considerable flow velocity of over 20 cm per second. “It’s remarkable how much blood flows through our vessels and at such high speed,” Landers pointed out. “Our navigation system must be able to withstand all of that.”
When the microrobot reaches a junction in the vessels through which it would be difficult to maneuver, in-flow navigation comes into play. The magnetic gradient is directed against the wall of the vessel in such a way that the capsule is carried along into the correct vessel.
By integrating these three navigation strategies, the researchers could maintain effective control over the microrobots across various flow conditions and anatomical scenarios. In more than 95% of the cases tested, the capsule successfully delivered the drug to the correct location.
To assess the microrobots and their navigation in a realistic environment, the researchers developed silicone models that accurately replicate the vessels of patients and animals. These vessel models are so realistic that they are now being used in medical training and are being marketed by ETH spin-off Swiss Vascular. “The models are crucial for us, as we practiced extensively to optimize the strategy and its components. You can’t do that with animals,” explained Pané. In the model, the researchers were able to target and dissolve a blood clot.
After numerous successful trials in the model, the team sought to demonstrate what the microrobot could achieve under real clinical conditions. First, they were able to demonstrate in pigs that all three navigation methods worked and that the microrobot remains clearly visible throughout the entire procedure. The investigators then navigated microrobots through the cerebral fluid of a sheep.
“This complex anatomical environment has enormous potential for further therapeutic interventions, which is why we were so excited that the microrobot was able to find its way in this environment too,” Landers noted. “In vivo experiments conducted with an ovine model demonstrated the platform’s ability to operate within anatomically constrained regions of the central nervous system,” the investigators stated in their paper. “Furthermore, in a porcine model, all locomotion strategies were validated under clinical conditions, confirming precise microrobot navigation within the cerebrovascular system and highlighting the system’s compatibility with versatile in vivo environments.”
In addition to treating thrombosis, these new microrobots could also be used for localized infections or tumors. At every stage of development, the research team has remained focused on their goal, which is to ensure that everything they create is ready for use in operating theaters as soon as possible. The next goal is to look at human clinical trials. “The use of materials that have been FDA approved for other intravascular applications, coupled with the modular design of the robotic platform, should simplify translation and adaptability to a range of clinical workflows,” the authors concluded. Speaking about what motivates the whole team, Landers said, “Doctors are already doing an incredible job in hospitals. What drives us is the knowledge that we have a technology that enables us to help patients faster and more effectively and to give them new hope through innovative therapies.”