A brain–computer interface developed by neurotechnology firm Paradromics contains electrodes that record from neurons in the cerebral cortex and then convert the information into intended speech patterns.Credit: Paradromics
Paradromics, a neurotechnology developer, announced today that the US Food and Drug Administration (FDA) has approved a first long-term clinical trial of its brain–computer interface (BCI). Early next year, the company — one of the closet rivals to Elon Musk’s neurotechnology firm Neuralink — will implant its device in two volunteers who were left unable to speak owing to neurological diseases. It has two goals: to ensure the device is safe; and to restore a person’s ability to communicate with real-time speech.
“We’re very excited about bringing this new hardware into a trial,” says Matt Angle, chief executive of Paradromics, which is based in Austin, Texas.
Paradromics’ BCI is a roughly 20 x 20 millimetre grid of thin, stiff, platinum-iridium electrodes that penetrate the surface of the cerebral cortex to record from individual neurons around 1.5 mm deep. This is then connected by wire to a power source and wireless transceiver implanted in an individual’s chest.
Initially, the two volunteers will each have one electrode array implanted in the area of the motor cortex that controls the lips, tongue and larynx, Angle says. Neural activity will then be recorded from this region as the study participants imagine speaking sentences that are presented to them. Following previous work by researchers who are now collaborating with Paradromics1, the system learns what patterns of neural activity correspond to each intended speech sound.
When participants imagine speaking these neural patterns will be converted into text on a screen for participants to approve, or into a real-time voice output based on old recordings of participants’ own voices.
This is the first BCI clinical trial to formally target synthetic-voice generation. “Arguably, the greatest quality of life change you can deliver right now with BCI is communication,” Angle says.
Elon Musk’s Neuralink brain chip: what scientists think of first human trial
The trial will also explore whether the system can reliably detect activity from imagined hand movements that would enable an individual to control a computer cursor. Depending on initial results, the trial could extend to ten volunteers with these participants receiving two cortical implants to increase signal richness and to access other brain areas, says Angle.
“I’m very curious. It’s an exciting step,” says Mariska Vansteensel, a BCI researcher at the University Medical Center Utrecht in the Netherlands. “For the field to move forward towards clinical applications, a fully implantable system is the only way to go.”
Neuralink rivals
Paradromics is now one of several companies testing an implanted BCI system that is similar to what it intends to market in a long-term trial. Several more companies hope to follow suit soon.
Furthest along is Synchron, a BCI company in New York City, and its device Stentrode. By incorporating 12–16 electrodes into a device that is based on an endovascular stent, the Stentrode records the average activity of a neuronal population from inside a blood vessel. Initial trials utilized a vessel in the motor cortex to allow users to select onscreen options by imagining that they are moving their foot.
Neuralink has prioritized obtaining high-bandwidth signals by recording outputs from many single neurons. Its BCI consists of 64 flexible polymer threads, each with 16 recording sites. In the company’s initial trial, it targeted the region of the motor cortex that controls the hand. Users controlled computers and robotic hands with their thoughts, and the company has regularly reported that it is able to transfer information from people’s brains to computers at record-breaking speeds.
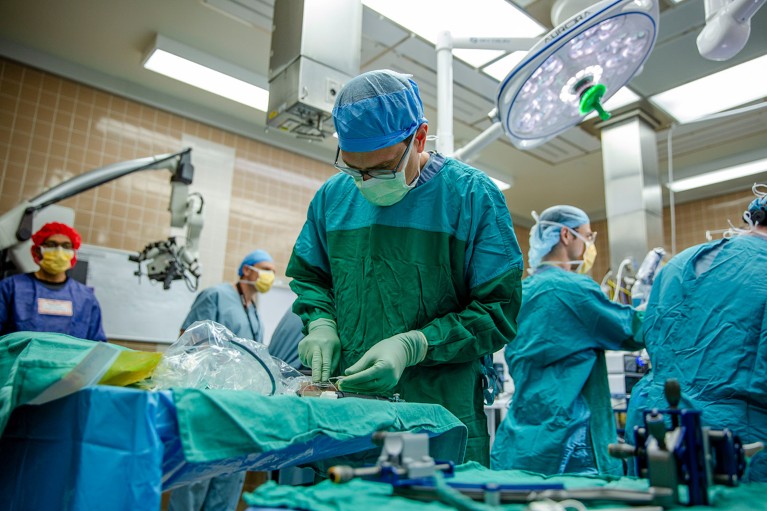
A clinical trial will assess the safety and durability of Paradromics’ brain–computer interface after it has been surgically implanted into the brains of two volunteers.Credit: University of Michigan
Other systems in development include several BCIs that place high-density electrodes on the brain’s surface to record the average activity of underlying neuronal populations.
As long-term trials now progress, clinicians and neuroscientists will be watching and comparing devices. Vansteensel says she hopes companies will be fully transparent and that in time they will make their devices available to academics to conduct independent studies.


