Symptoms from HIV infection can currently be managed with lifelong treatment. However, there is no cure and new targets are needed due to the development of drug resistance. A promising treatment target is the retroviral integrase enzyme which has two roles in the viruses replication cycle, each requiring a different structure.
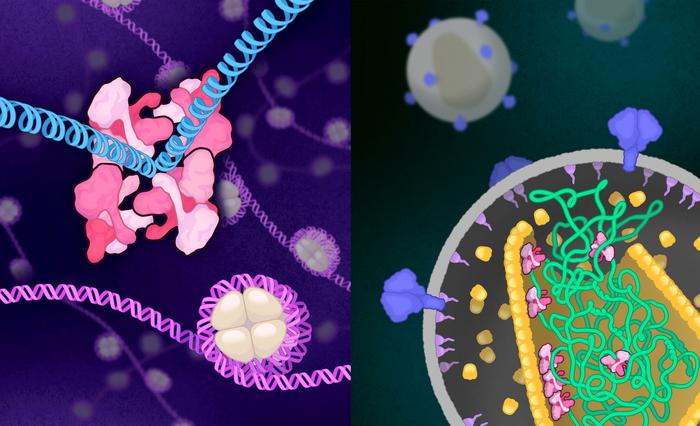
During early stages of infection, integrase functions within an oligomeric “intasome” assembly to catalyze viral DNA integration into host chromatin. During late stages of infection, tetrameric integrase binds viral RNA and orchestrates the condensation of ribonucleoprotein complexes into the capsid core. But the molecular architectures of HIV-1 integrase assemblies that mediate these distinct events has remained unknown.
Now, researchers at the Salk Institute have captured the structural changes between these functions for the first time, creating novel 3D models of integrase in both roles.
“We are just now finding that these integrase proteins that we have studied for years perform unexpected functionalities, like interacting with RNA,” says Dmitry Lyumkis, PhD, associate professor at Salk. “Determining how integrase interacts with RNA will help us better understand this new role and inform the design of novel and more effective HIV therapeutics.”
This work is published in Nature Communications in the paper, “Oligomeric HIV-1 integrase structures reveal functional plasticity for intasome assembly and RNA binding.”
Integrase performs the DNA-insertion process, which is a hallmark of the retroviral replication cycle, making the protein an obvious target for HIV-1 drugs like Dolutegravir. However, HIV-1 evolves rapidly and is prone to developing drug resistance. In 2023, Lyumkis discovered how integrase adapts its structure to evade Dolutegravir.
Instead of targeting integrase during DNA insertion, future drugs could instead target integrase during its recently discovered second role: interacting with the newly produced viral RNA as it’s packaged into nascent viruses that have left the cell.
“Very little is known about what integrase is doing in the later stages of HIV replication,” says Tao Jing, PhD, a postdoctoral researcher in the Lyumkis lab. “Our use of cryo-electron microscopy to discover the architecture of integrase during this mysterious period is a significant step for HIV research.”
The researchers used cryo-electron microscopy to collect two distinct integrase structures: 1) the form that integrates the viral DNA into the host cell’s genome, and 2) the form that likely interacts with the newly produced viral RNA later in the HIV replication process.
First, they determined integrase’s architecture as it forms the “intasome”—a special assembly of proteins and viral DNA. In this intasome form, integrase is made up of four identical four-part complexes that connect to create one 16-part complex.
Second, they determined integrase’s protein architecture as it interacts with RNA. At this point, the protein ditches the giant sixteen-part complex in favor of a simpler, smaller four-part complex. Based on this four-part structure, the team has an idea of how integrase might interact with RNA, and they are planning follow-up studies to confirm their suspicions.
More specifically, the authors write that they “determined cryo-EM structures of wildtype HIV-1 [integrase] tetramers and intasome hexadecamers.” The structures, they add, “unveil a remarkable plasticity that leverages [integrase] C-terminal domains and abutting linkers to assemble functionally distinct oligomeric forms. Alteration of a newly recognized conserved interface revealed that both [integrase] functions track with tetramerization in vitro and during HIV-1 infection.”
Integrase is a highly adaptable protein, capable of building up into a 16-part complex, then breaking back down into a four-part complex. Lyumkis says this flexibility is surprising, and though some of the structural changes are subtle, they can make substantial differences in the drug development process.
“We’ve created the first blueprints for integrase’s structure during these crucial steps in HIV replication,” says Zelin Shan, PhD, a postdoctoral researcher in the Lyumkis lab. “Now we can use those blueprints to design new drugs that suit this structure and disrupt the destructive HIV-1 invasion and replication process.”