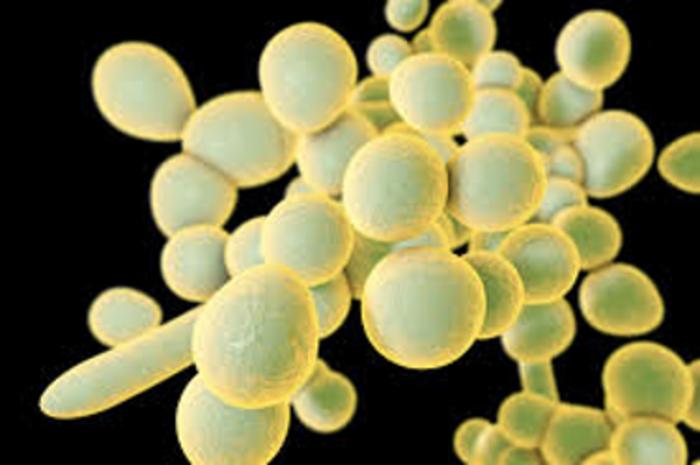
Candida species are an increasing cause of invasive fungal disease, particularly in hospitalized and immunocompromised populations. Not only is the arsenal of antifungal medicines and vaccines limited, but emerging resistance—most notably among Candida auris and Candida albicans—contributes to increasing morbidity and mortality rates. There is an urgent need for new therapeutic strategies targeting Candida infections.
Now, the Lundquist Institute (TLI) and Vitalex Biosciences announce that they will move forward in the development of the second-generation fungal vaccine candidate known as VXV‑01—up to and including Phase I clinical evaluation. VXV-01 is built on the Therapeutic & Laboratory Immunology technology platform developed at TLI. A dual-antigen fungal vaccine candidate, VXV-01 is designed to elicit robust immunity against key opportunistic and hospital-associated Candida and Gram-negative bacterial pathogens.
With this funding, the program is positioned to begin manufacturing and other preparations necessary to initiate two Phase I clinical trials. The contract, awarded by National Institutes of Health NIH/NIAID, provides up to U.S. $40 million of non-dilutive funding to Vitalex and its collaborator Appili and for the comprehensive development of VXV-01.
“Securing this contract is a pivotal moment for our team,” said Ashraf Ibrahim, PhD, principal investigator and VXV-01 program lead at TLI and CEO of Vitalex. “By putting VXV-01 into development through human trials, we are significantly increasing our chances of making this vaccine a reality. It is the culmination of years of work on our TLI technology platform and reflects our commitment to tackling high-impact infectious disease challenges.”
This contract represents a significant milestone for The Lundquist Institute’s vaccine research program. Founded in 1952, TLI is a nonprofit research organization at the heart of Los Angeles County’s life sciences ecosystem.
Vitalex, which has an exclusive option to license VXV-01 from TLI for commercial development, is a start-up company created to further the development of technologies discovered in the Ibrahim lab at TLI. The lab focuses on the development of a dual antigen vaccine that targets hospital-acquired infections caused by multidrug resistant Candida albicans, Candida auris, and Gram-negative bacteria including those caused by Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae. Appili Therapeutics is currently advancing a diverse range of anti-infectives, including an FDA-approved ready-made suspension of metronidazole for the treatment of antimicrobial resistant infections, a vaccine candidate to prevent tularemia, a serious biological weapon threat, and a topical antiparasitic for the treatment of cutaneous leishmaniasis.