Sponsored content brought to you by
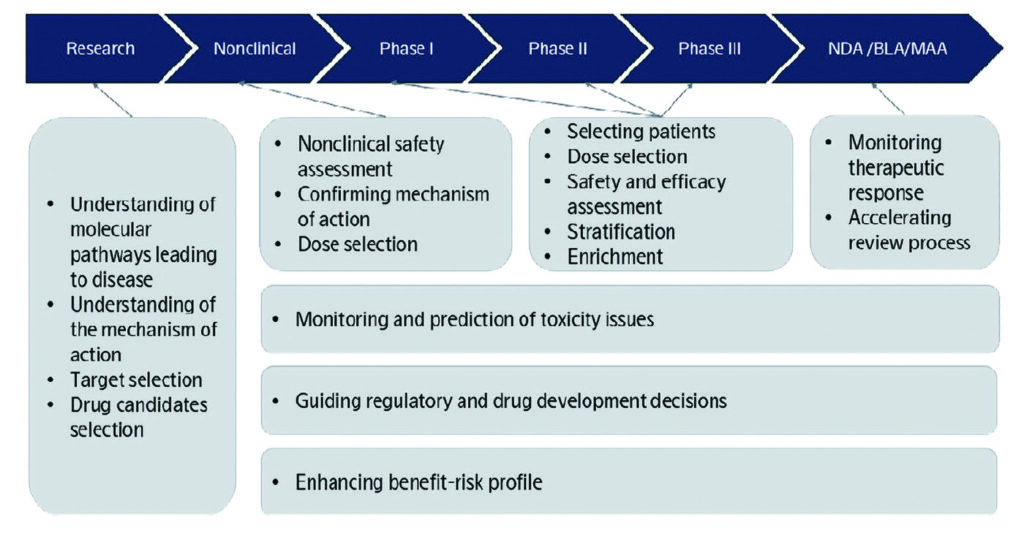
Drug development remains a high-risk, capital-intensive endeavor, with average costs exceeding $1 billion per approved therapy and late-stage failure rates remaining high. As pressure mounts to accelerate timelines, reduce costs, and improve success rates, biomarkers are increasingly viewed not only as discovery research tools, but as strategic assets. Enabled by advances in protein biomarker technologies, biomarkers can support smarter decision-making across the drug development lifecycle—from target selection to clinical trials—making programs more efficient, data-driven, and precise.1
Biomarker insights improve drug target selection
Poor target selection is a significant contributor to late-stage clinical failure, often resulting from insufficient biological validation early in development. These failures carry enormous scientific and financial costs, with late-stage attrition estimated to exceed $1.3 billion per drug.2 Protein biomarkers offer actionable insights into disease biology, enabling teams to select targets with stronger mechanistic relevance and a higher probability of success.3
Key biomarker-driven strategies for improving target selection include:
Combining genomics, transcriptomics, and proteomics delivers a more holistic view of disease biology. While genomics identifies risk, proteomics captures real-time functional changes that drive disease. Large-scale efforts such as the UK Biobank Pharma Proteomics Project (UKB-PPP) demonstrate how proteogenomic integration can reveal novel disease subtypes and improve prediction models across more than 60 diseases, strengthening target identification and validation4.
Proteomic data from clinical trials can inform upstream R&D decisions by revealing how drugs modulate biological pathways in real patients. This feedback loop supports better early target prioritization and enables faster discontinuation of non-performing candidates—an approach that reduces downstream risk and conserves resources.
Biological samples are often scarce, particularly in rare diseases or invasive biopsy settings. High-throughput proteomic platforms enable thousands of protein measurements from minimal sample volumes, generating richer datasets while preserving valuable patient material. This maximization of data per sample supports deeper biological insight and better-informed discovery decisions.
Biomarker insights support data-driven decision-making
Beyond discovery, protein biomarkers are transforming clinical trials by enabling real-time insights into efficacy, safety, and patient heterogeneity. These capabilities support objective, evidence-based decisions that optimize trial design and execution.
Key biomarker-driven strategies for mitigating development risk include:
- Early efficacy evaluation:
Proteomic biomarkers provide direct measures of target engagement and pathway modulation, allowing teams to assess whether a drug is working as intended early in development. This enables rapid go/no-go decisions and supports a fail-fast strategy that saves time and cost.
Proteomics can uncover molecular differences within clinically similar patient populations, enabling more precise stratification and better matching of therapies to biology. This improves trial outcomes and supports precision medicine approaches5,6.
Blood-based protein biomarkers offer alternatives to invasive biopsy endpoints, particularly in liver and kidney diseases. These non-invasive measures reduce patient burden, improve recruitment, and enable faster and more frequent trial readouts.
As biomarkers become increasingly integrated into drug development, their role is shifting from supportive to strategic. By linking disease biology, target engagement, and clinical outcomes, biomarkers provide a cohesive framework for reducing development risk, improving decision-making, and increasing the likelihood of successful therapeutic advancement3.
References
- Gromova M, Vaggelas A, Dallmann G, Seimetz D. Biomarkers: opportunities and challenges for drug development in the current regulatory landscape. Biomark Insights. 2020;15:1177271920974652. doi:10.1177/1177271920974652
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20-33. doi:10.1016/j.jhealeco.2016.01.012
- Wagner JA. Strategic approach to fit-for-purpose biomarkers in drug development. Annu Rev Pharmacol Toxicol. 2008;48:631-651. doi:10.1146/annurev.pharmtox.48.113006.094611
- Carrasco-Zanini J, Pietzner M, Davitte J, et al. Proteomic signatures improve risk prediction for common and rare diseases. Nat Med. 2024;30:2489-2498. doi:10.1038/s41591-024-03142-z
- Parra ER, Villarroel-Espindola F, Behrens C, et al. Multi-omics analysis reveals immune features associated with immunotherapy benefit in patients with squamous cell lung cancer from Phase III Lung-MAP S1400I trial. Clin Cancer Res. 2024;30(8):1655-1668. doi:10.1158/1078-0432.CCR-23-0251
- Zannad F, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur Heart J. 2022;43(48):4991-5002. doi:10.1093/eurheartj/ehac495
 To read the full whitepaper on which this article is based, scan the QR code or visit go.olink.com/insight-to-impact.
To read the full whitepaper on which this article is based, scan the QR code or visit go.olink.com/insight-to-impact.


