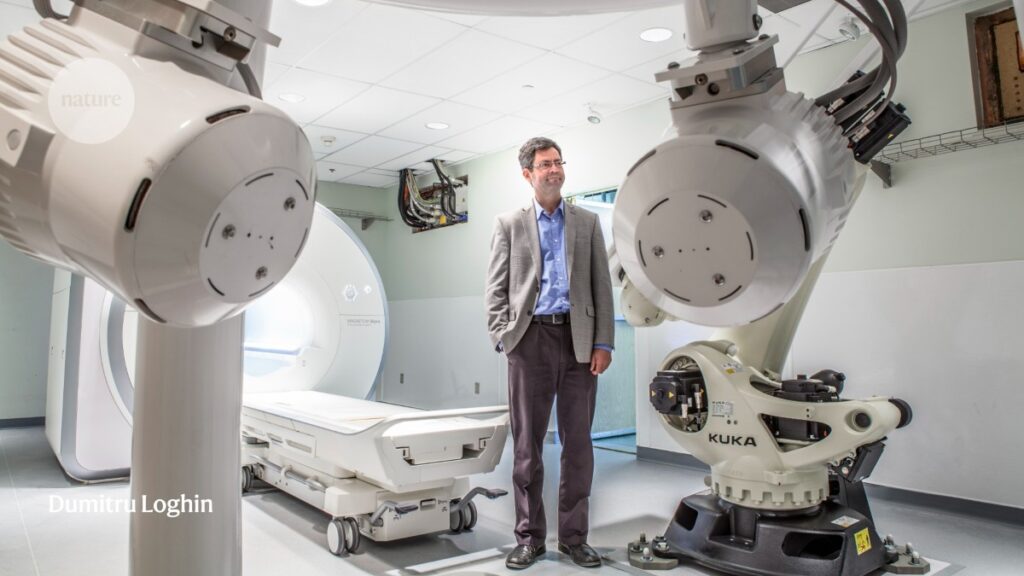
Sylvain Martel at Polytechnique Montréal uses magnetic-resonance-imaging scanners to steer drug-loaded nanorobots in pigs.Credit: Dumitru Loghin
The live pig in the magnetic resonance imaging (MRI) machine was a common sight for Sylvain Martel, a nanorobotics researcher at Polytechnique Montréal in Canada, by the time he stepped into the imaging suite one evening in 2017. By then, Martel and his colleagues had spent more than a decade refining swarms of tiny robots that they could steer through the animals using the machine’s magnetism. The hope was that such nanorobots could one day be a delivery vehicle for cancer-busting drugs. That evening, the group was exploring the effect of gravity, testing whether changing the pig’s positioning in the machine could help the nanobots to navigate the many branching arteries to the liver.
Nature Spotlight: Robotics
As the team watched the screen, the robots gradually shifted towards the liver, amassing there as a bright, flickering cloud. Compared with previous trials, in which the pigs were lying flat on their backs, placing them inside the MRI at a slight downward angle prompted an almost threefold increase in the number of robots that arrived at their destination. Martel says that even though the successful experiment only presaged more work, it nevertheless felt like a culmination of years of trial and error.
“All these years, we’d been diligently showing different steps of the process. We refined the technology, we showed that the robots could carry and administer drugs and now we’d shown that we could move them efficiently across human-scale distances,” he says. Getting to this stage “took a long time, but it also feels like the next steps should go much faster”.
The work of Martel and his colleagues has taken scientists closer to deploying nanorobotic technology in humans — a threshold that, when crossed, could one day revolutionize the diagnosis, monitoring and treatment of many cancers. Already, late-stage research in animal models has demonstrated how nanorobots can assist clinicians with biopsies and surgeries, serve as screening agents for hard-to-detect cancers, starve tumours of oxygen and deliver cancer-killing drugs and vaccines. Cancer, in particular, is a field ripe for nanorobotics innovation.
Borrowing from nature
Nanorobots are loosely defined by their size — typically less than 100 nanometres — and their ability for directed or autonomous motion. This movement distinguishes them from technologies such as nanocarriers, which can transport molecular cargo but do so passively. Even slightly larger microrobots, around 1 millimetre in size, often rely on nanotechnology, for example in their motors, or exploit physical phenomena occurring at the nanoscale. Therefore, researchers often lump the two under the combined term micro-nanorobots (MNRs).
Rudimentary versions of these robots appeared in the 1990s, offering proof-of-concept that researchers could indeed create microscale robots. Roughly two decades later, the first MNR made its way into cancer research, when a team in South Korea developed a “bacteriobot” capable of detecting solid tumours by homing in on chemical gradients given off by the cancerous cells1. MNRs are often broken down into two categories, inorganic and organic, on the basis of the materials used to construct them. Inorganic robots might include metals such as silver, gold or iron, and organic robots are often built using scaffolds of DNA or proteins. Both types can be manipulated using forces such as magnetism, light and acoustics or by chemical and catalytic reactions that propel them towards their destination.

Tiny grippers (pictured) can take cancer biopsies.Credit: Evin Gultepe, Gracias Lab, JHU
Björn Högberg, a biophysicist at the Karolinska Institute in Stockholm, says that the methods for building nanorobots have been refined over time, particularly the fabrication of their components. Högberg says much of his work focuses on a technique called DNA origami, in which scientists treat DNA “not as a genetic material, but as a construction material” capable of building intricate 2D and 3D structures. “There are now many clever examples of using origami to conceal things like tumour-targeting ligands or drugs, where it exposes its cargo in the right place and at the right time,” he says.
Robots demonstrate principles of collective intelligence
Increasingly, researchers are creating what are known as biohybrid robots, which introduce synthetic elements into biological entities such as bacteria, algae and sperm. Scientists quickly realized that nature had already designed many of the features that make for a successful nanorobot — including whip-like flagella for motion, and sensors for detecting gradients. “A bacterium already has a motor, with all the components we’d recognize,” says Martin Pumera, a nanorobotics researcher at the Central European Institute of Technology in the Czech Republic. “What we’re pursuing now is the ideal combination of both worlds, the synthetic one and the biological one.”
Such advances have given researchers more control over robots’ speed and orientation and even enabled them to develop devices that can swap between propulsion mechanisms. Martel, for example, used a marine bacterium to create a nanobot that can be moved to the vicinity of a tumour using magnetism, and can then switch to an on-board sensor that detects oxygen gradients as it draws near2. Because the tumour microenvironment has low oxygen, “it creates a beacon for these robots to seek out”, Martel says.
Coming for cancer
With these building blocks in place, scientists are now applying nanorobotic technology across the cancer-research spectrum, using nanobots to investigate at least a dozen cancer types. Joseph Wang, a nanoengineer at the University of California, San Diego, says that cancer might be the field with the most to gain from these precision-medicine tools, in part because of how challenging cancer can be to root out and how invasive existing treatments can be. “There’s a desire among patients for treatments that don’t require surgeries or painful injections, and I think nanorobots have a lot to offer here,” he says.
Today, the largest strides in cancer-based nanorobotics research are in targeted drug delivery — offering a notable improvement over conventional treatments, such as chemotherapy and radiation therapies, that are broad-acting with severe side effects. And the development of targeted therapies is the goal not just of cancer research, but of precision medicine more broadly.
Why we should limit the autonomy of AI-enabled weapons
One promising therapy is the use of nanorobots to deliver the enzyme thrombin to tumours, to effectively starve cancer cells of oxygen by coagulating the blood that feeds them. In China, researchers have used DNA origami to create nanorobots that bind to nucleolin, a protein expressed on vascular cells that deliver blood to a tumour3. The binding also serves as a trigger to change the conformation of the origami and release the thrombin. This therapy has so far been tested in mouse models of an aggressive form of breast cancer, in which it dampened tumour growth, kick-started death of cancerous cells, and prevented cancer spread (metastasis).
Mariana Medina-Sánchez, a nanobiosystems researcher at the Basque Nanoscience Cooperative Research Center in San Sebastian, Spain, focuses on cancers that affect the female reproductive system. She says that researchers often develop MNRs that are based on the specifics of the system they’re working in, as she has done for the reproductive system. Her MNR is a biohybrid one built from a sperm cell and a magnetic 3D-printed structure that can be guided by magnetism and release the sperm at the target4. As well as its powerful tail, a sperm cell contains enzymes for fusing with cell membranes. Medina-Sánchez has co-opted that ability, enabling the MNR to bind to synthetic cervical and ovarian tumours, where they then release drugs.
“What we’re finding is that we can load them up with anti-cancer drugs — even combination therapies — without affecting their viability, which makes them ideal,” she says. “In fact, their tail-beating is so robust” that they also do physical damage to the tumour simply by disrupting the cell environment. The biohybrid sperm also have a natural ability to dampen immune responses by displaying certain proteins, making them less likely to damage their host.

Grippers shown next to an 18-gauge needle for comparison.Credit: Evin Gultepe, Gracias Lab, JHU