The educational content in this post, elaborated in collaboration with Biocodex Microbiota Institute, was independently developed and approved by the GMFH publishing team and editorial board.
Uncovering functional resilience in the gut microbiome
Antibiotic therapy, though essential in modern medicine, remains one of the most powerful disruptors of the human gut ecosystem. Beyond depleting microbial diversity, antibiotics dismantle the intricate metabolic network that sustains intestinal homeostasis. Restoring these functions—rather than merely recolonizing bacteria—has emerged as a central challenge in microbiome therapeutics1.
The recent study by Huang et al. (2025, Gut Microbes)2 for the first time demonstrates that the probiotic yeast Saccharomyces boulardii CNCM I-745 not only stabilizes gut microbiota composition during antibiotic exposure but also restores its metabolic activity, safeguarding the biochemical dialogue between microbes and host.
Using a combination of advanced in vitro gut models, metagenomics, metabolomics, and human immune assays, the authors dissected the yeast’s direct effects on the microbiota, independent of the host. This broad and successful strategy can now be used as a model to address functionally restorative probiotics.
From taxonomy to function: a shift in microbiome research
Traditional probiotic studies have focused on microbiota composition—tracking how specific taxa increase or decrease under treatment. However, the real measure of a healthy gut ecosystem lies in function: the ability of microbes to ferment dietary substrates, synthesize beneficial metabolites, and regulate immune balance.
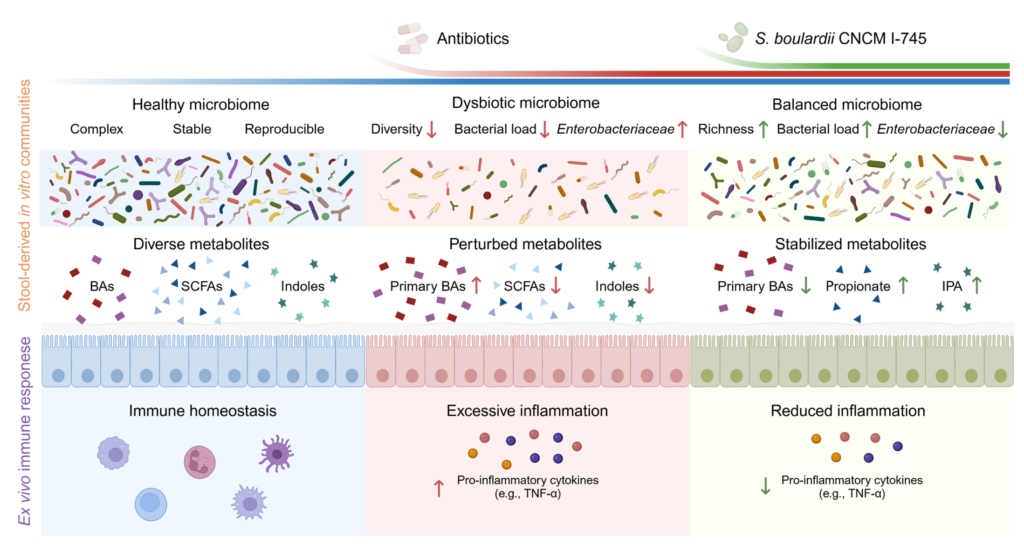
Huang et al. applied quantitative microbiota profiling and shotgun metagenomics to capture both compositional and functional dynamics of the microbiome under antibiotic pressure. In two in vitro models of human gut microbiota culture models, MiPro (static) and SHIME® (dynamic), the team observed that amoxicillin/clavulanic acid (AMC), broadly used antibiotics in human medicine, markedly reduced bacterial biomass, diversity, and metabolic output. Supplementation with S. boulardii CNCM I-745 mitigated these effects, maintaining bacterial load and restoring key metabolic pathways related to energy and carbohydrate metabolism.
This finding is critical: it shows that preserving microbial biomass, rather than reshaping community composition, is sufficient to maintain the ecosystem’s functional integrity. The yeast acted as a stabilizing force, allowing resident microbes to continue “doing their job” under antibiotic stress.
Restoring metabolic communication: propionate and indole-3-propionic acid
A highlight of the study lies in its detailed metabolomic analysis. Antibiotic exposure disrupted the production of short-chain fatty acids (SCFAs) and tryptophan-derived metabolites, molecules central to host-microbiota communication.
Saccharomyces boulardii CNCM I-745 supplementation restored propionate and indole-3-propionic acid (IPA) production, two metabolites with distinct yet complementary roles. Propionate, a SCFA mainly produced by Bacteroides species, modulates mucosal immunity and promotes regulatory T cell development. IPA, derived from tryptophan metabolism, reinforces epithelial barrier integrity and dampens NF-κB–mediated inflammation.
Interestingly, S. boulardii itself displayed minimal intrinsic metabolic activity under anaerobic conditions, suggesting that the observed effects stem from ecological facilitation, the yeast supports the recovery and activity of native bacteria rather than acting as a direct metabolic producer. This subtle yet powerful mechanism may represent a defining feature of probiotic yeasts.
Reducing the pro-inflammatory potential of antibiotic-disturbed microbiota
To explore host relevance, the authors exposed human immune cells (PBMCs) and intestinal mucosal explants from healthy subjects to microbiota-derived supernatants from the MiPro and/or the SHIME® model. Microbiota exposed to antibiotics alone triggered strong pro-inflammatory cytokine secretion, including TNF-α, IL-6, and MCP-1. In contrast, exposure to S. boulardii–supplemented microbiota markedly reduced these inflammatory signals. Of note, S. boulardii supernatant itself displayed minimal effects on cytokines production by human cells.
These findings suggest that S. boulardii exerts indirect immunomodulatory effects, mediated through the microbiome’s restored metabolic activity. By maintaining the production of anti-inflammatory metabolites such as SCFAs and IPA, the yeast contributes to an environment conducive to immune tolerance and mucosal repair.
This mechanistic clarity strengthens the rationale for exploring microbial metabolite, driven therapies as adjuncts to antibiotics and expands our understanding of the microbiota–metabolite–immune axis in gut resilience.
Scientific and methodological advances
One of the most valuable contributions of this research lies in its methodological rigor. By integrating static (MiPro) and dynamic (SHIME®) in vitro systems with immune assays on human cells, the team demonstrated how S. boulardii’s effects can be traced across scales—from microbial metabolism to host immune response.
Importantly, in in vitro models of human gut microbiota culture, the use of human-derived microbiota and the exclusion of host confounders (e.g., diet, stress, medication, host cells) allowed precise observation of direct microbe–microbe interactions. This approach establishes a robust experimental framework for future mechanistic studies on probiotics and microbiome therapeutics.
Toward a new definition of probiotic efficacy
The findings of Huang et al. carry broad implications for the microbiome field. They underscore that effective probiotics need not dramatically alter microbial composition to exert meaningful health effects. Instead, sustaining microbial biomass and preserving metabolic function may be the true determinants of resilience.
In this view, S. boulardii CNCM I-745 exemplifies a next-generation probiotic—one that safeguards microbial function, mitigates antibiotic-induced dysbiosis, and contributes to host immune balance without competing with native bacteria or antibiotics themselves.3
As microbiome research continues to evolve toward precision and function, this study provides a compelling model for evaluating probiotics through a systems biology lens, linking microbial ecology, metabolism, and immunology in a unified framework.
References:
- Guarner F, Bustos Fernandez L, Cruchet S, et al. Gut dysbiosis mediates the association between antibiotic exposure and chronic disease. Front Med. 2024; 11:1477882. doi: 10.3389/fmed.2024.1477882.
- Huang Z, Brot L, Fatouh R, et al. Saccharomyces boulardii CNCM I-745 mitigates antibiotic-induced gut microbiome functional alterations independently of the host. Gut Microbes. 2025; 17(1):2575924. doi: 10.1080/19490976.2025.2575924.
- Waitzberg D, Guarner F, Hojsak I, et al. Can the evidence-based use of probiotics (notably Saccharomyces boulardii CNCM I-745 and Lactobacillus rhamnosus GG) mitigate the clinical effects of antibiotic-associated dysbiosis? Adv Ther. 2024; 41(3):901-914. doi: 10.1007/s12325-024-02783-3.