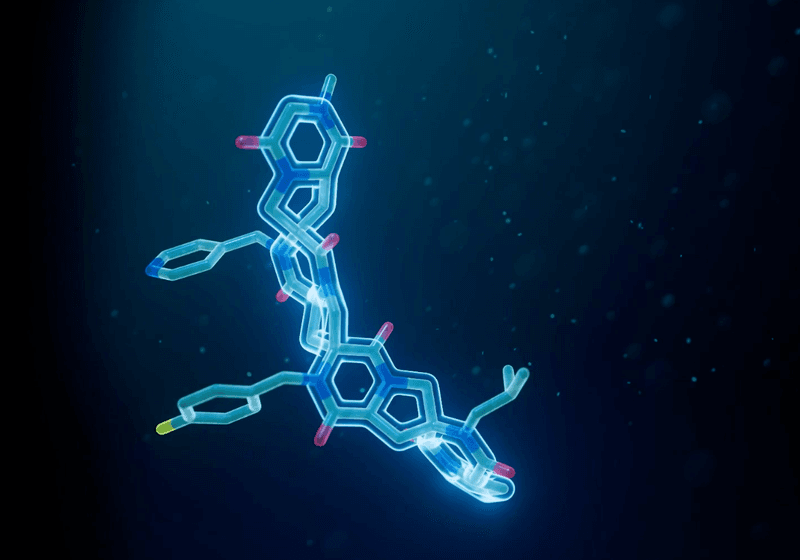
New DNA-encoded macromolecules snap together like molecular Lego blocks, providing precise control over shape and function in drug design.
Traditional approaches to drug development face trade-offs. Small molecules are cell permeable and easy to manufacture, but they often suffer from poor specificity and off-target effects. Antibodies and peptides tightly bind their targets but are large, costly to produce, and difficult to administer. To address these challenges, scientists at the startup company ThirdLaw Molecular developed synthetic molecules called Spiroligomers™, which combine the best properties of small molecules, peptides, and antibodies.
Designing novel peptides for therapeutics has proven difficult, as the myriad complex ways that proteins fold are hard to predict. “There’s rotation between every pair of amino acids, so you get these long chains that have to fold into a three-dimensional shape,” Christian Schafmeister, the founder and president of ThirdLaw Molecular, explained. “I thought, if the problem is rotatable bonds, why don’t we make building blocks that we link, not through one bond, but through two?” These starting materials are rings that connect like Lego blocks, forming ladder-shaped backbones that can be decorated with functional side chains to attain the desired shape and activity.
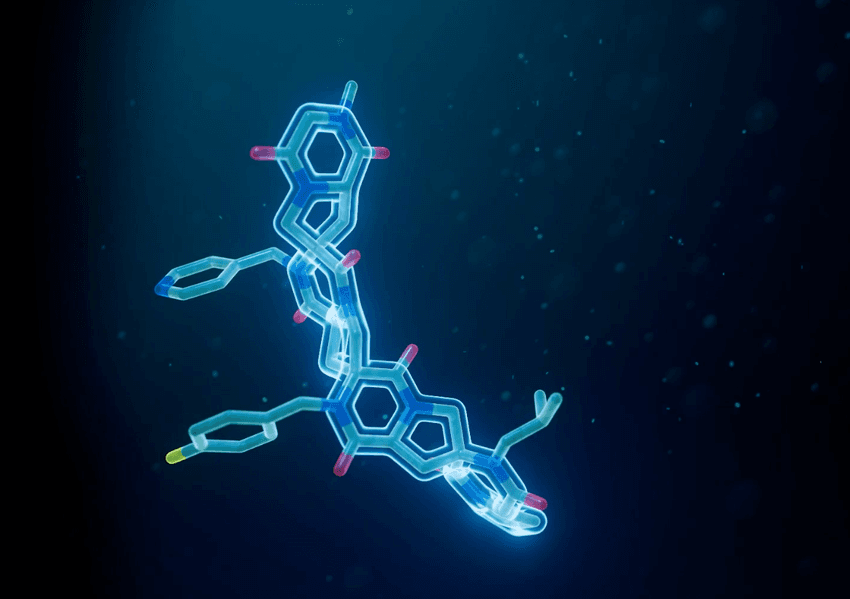
ThirdLaw Molecular’s Spiroligomer™ molecules combine the best of small molecules, peptides, and antibodies to accelerate drug discovery.
Maurice Murphy
Making fused ring systems has historically been a challenging process, taking scientists years to make scant quantities. For Spiroligomers, ThirdLaw Molecular’s scientists developed a scalable process that starts with hydroxyproline, an inexpensive ringed amino acid derived from cartilage. Their approach enables multi-kilogram production of Spiroligomer building blocks that can be combined in countless ways to generate new drug candidates. “The real potential of this technology is that it gives us a way to create large molecules with control over their shape on the molecular scale,” Schafmeister said.
In January 2025, ThirdLaw Molecular unveiled a library of 4.5 billion Spiroligomer™ macromolecules. Each Spiroligomer has an identifying DNA barcode, allowing high-throughput screening for binding activity. The company is now partnering with pharmaceutical companies, with hopes of using the library to develop drugs that bind targets with antibody-like precision, permeate cells easily, and can be delivered orally.
Beyond drug discovery, Schafmeister envisions developing enzyme-like Spiroligomers capable of catalyzing reactions, breaking down pollutants, or producing raw materials. “What’s driven me all this time is to create this technology that can really impact the world,” he said. “All the things biology does to make nature work, we can now do with these building blocks.”
Check out all of The Scientist‘s Top Innovation winners of 2025.