When Aaron Phillips, PhD, was in graduate school, a lab member who became a close friend had a spinal cord injury (SCI). “He shared with me the serious blood pressure instability he faced every day,” Phillips told Inside Precision Medicine. “His experience sparked my curiosity and motivated me to develop and utilize my skills to understand and solve this problem.”
Blood pressure instability is a hidden but debilitating consequence of SCI, causing daily dizziness, blurred vision, fatigue, and fainting episodes triggered by standing, eating, or even light exertion. These hypotensive complications limit rehabilitation, increase stroke and heart disease risk, and reduce independence and quality of life, and current treatments, such as abdominal binders, compression stockings, high-salt diets, and midodrine, rarely provide consistent relief.
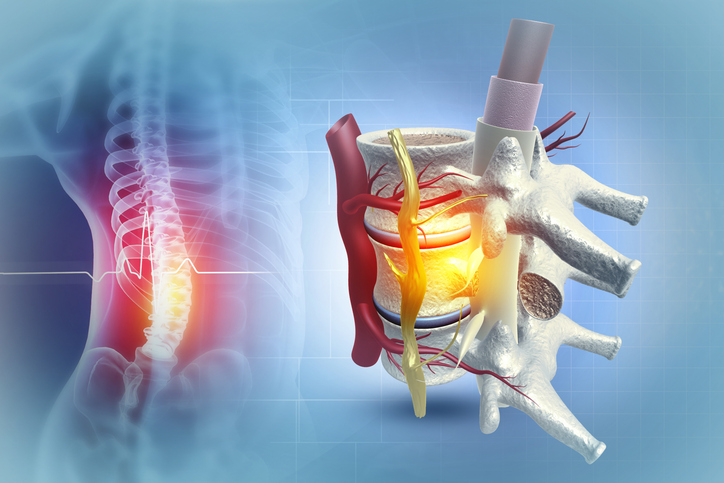
Phillips, now an associate professor at the University of Calgary, and an international team of researchers have developed an implantable neurostimulation device that stabilizes the body’s ability to maintain stable blood pressure (i.e., hemodynamics), which is essential for neurological recovery. Together with researchers from the University of Calgary, the Swiss Federal Institute of Technology (EPFL), Lausanne University Hospital (CHUV), and the University of Lausanne (UNIL), Phillips created a biomimetic epidural electrical stimulation (EES) device—a small implant that sends gentle electrical pulses to a specific part of the spinal cord, copying the body’s natural signals. This study, published in Nature Medicine, paves the way for a larger clinical trial to confirm that this new approach could become a reliable therapy for people with spinal cord injuries who struggle with dangerously low blood pressure.

The same group, headed by French neuroscientist Gregoire Courtine, PhD, a professor at EPFL, also published a companion study in Nature. Phillips, elaborating on the back-to-back studies, said, “These studies show, for the first time, that precisely targeted epidural electrical stimulation can safely and durably stabilize blood pressure in people living with chronic spinal cord injury. [The Nature Medicine] paper defines the clinical burden of chronic hypotension and proves that stimulation of the last three thoracic segments corrects low blood pressure when standard treatments fail. The companion [Nature] paper maps the spinal circuits that trigger dangerous hypertensive episodes and reveals how stimulation engages overlapping neurons to prevent these crises. Together, they establish the mechanistic blueprint and clinical evidence needed to launch a pivotal device trial for blood pressure control after spinal cord injury.”
The underestimated burden of SCI hypotension
To understand the scope of the problem, researchers analyzed data from 1,479 people with chronic SCI. Among individuals with tetraplegia, 78% had been diagnosed with orthostatic hypotension, but over 90% still experienced symptoms despite treatment. A second survey of 254 people confirmed that virtually all respondents with tetraplegia experienced persistent hypotensive symptoms throughout the day. These findings, combined with evidence linking blood pressure instability to cardiovascular complications, provided a strong justification for new approaches—despite the need for a neurosurgical procedure.
“For people living with spinal cord injury, blood pressure instability is extremely common yet often untreated,” said Phillips. “Current clinical tools are limited, and most lack strong evidence of effectiveness. This contributes to increased mortality and reduced quality of life. In summary, there are no effective treatments for blood pressure instability, and that is unfortunate, as it can be devastating to quality of life and reduce lifespan.”
The team built on years of preclinical studies showing that EES could activate sympathetic circuits that regulate blood pressure. By mapping responses in rats, nonhuman primates, and humans, they identified the lower thoracic spinal cord as the “hemodynamic hotspot,” where clusters of sympathetic preganglionic neurons are densest.
In six volunteers with medically refractory orthostatic hypotension, researchers compared EES delivered over the lower thoracic hotspot versus the more commonly targeted lumbosacral segments. Lower thoracic stimulation consistently produced robust and reproducible blood pressure stabilization without inducing painful leg spasms—resolving a long-standing debate about where to stimulate for hemodynamic control.
A closed-loop implant tailored for blood pressure
Because existing stimulators were designed for pain management, the team engineered a purpose-built system. The implant communicates with a wearable hub that continuously monitors blood pressure and adjusts stimulation in real time, within 25 milliseconds. A redesigned paddle lead was optimized to cover the last three thoracic segments for full hotspot activation while avoiding side effects.
In new clinical studies across multiple sites, eight participants received the system or its components. All demonstrated immediate improvement in blood pressure stability during tilt-table tests. Over follow-up periods of up to two years, participants continued to use the system daily, discontinued other therapies, and reported better tolerance of postural changes, meals, and activities. The implanted system immediately and consistently raised and stabilized blood pressure in people with chronic spinal cord injuries. Objective measures—including increased norepinephrine levels, cerebral blood flow, and sympathetic nerve activity—confirmed physiological benefits. Over time, it reduced dizzy spells and other symptoms, so patients no longer needed their old treatments.
The team also discovered the exact spot where the stimulation needs to be applied: the lower thoracic region of the spinal cord—not the lower back area doctors had been targeting before. Participants also reported reduced symptom severity and improvements in quality-of-life scores. No device-related serious adverse events occurred across hundreds of stimulation sessions. Even a surgical team at a new clinical site, unfamiliar with the technique, successfully implanted and programmed the device, demonstrating its scalability.
These results provide the first durable evidence that closed-loop EES at the hemodynamic hotspot can control blood pressure in people with SCI. The authors conclude that the therapy’s safety, effectiveness, and ease of deployment warrant pivotal clinical trials. “Our [Nature Medicine] study shows that spinal cord stimulation can promote stable control of blood pressure,” said Phillips. “It extends neuromodulation into autonomic regulation in a rigorous, mechanism-grounded, translational manner.”
This study paves the way for a larger clinical trial to confirm that this new approach could become a reliable therapy for people with spinal cord injuries who struggle with dangerously low blood pressure. “The key next step is regulatory approval so that patients around the world can benefit, feel better in their daily lives, and live longer, healthier lives,” said Phillips. “Achieving this requires a pivotal clinical trial, which we are now planning with the support of ONWARD Medical, a company deeply embedded in the spinal cord injury community and founded by Gregoire Courtine and Jocelyne Bloch.”
If validated in larger studies, this targeted neuromodulation could transform care for the majority of people with SCI who live with persistent, disabling hypotension—offering not just symptom relief, but restored confidence in daily life.