Circulating tumor cells were first described in 1869 by Thomas Ashworth, an Australian pathologist who observed them in a peripheral blood sample taken from a patient with metastatic cancer.1 They have since been detected in a range of tumor types, including breast, prostate, colorectal, ovarian, lung, liver, gastric, and pancreatic cancers, as well as melanoma.2
Researchers and clinicians can use circulating tumor cells to study cancer in a non-invasive way, gaining valuable insights into the biology of tumors. In this article, we explore what these cells are, the challenges associated with studying them, and how they enable scientists to diagnose cancer early, predict clinical outcomes, understand metastasis, monitor the efficacy of treatments, and develop personalized medicines.
What Are Circulating Tumor Cells?
Circulating tumor cells, sometimes referred to as CTCs, are cells that have detached from primary tumors to circulate in the blood or lymphatic systems.3 These cells give rise to metastatic tumors, which are the most frequent cause of cancer mortality.4
Carolina Reduzzi, a cancer biologist at Weill Cornell Medical College, explained that a circulating tumor cell is an intact, living cell that serves as an accurate representation of the biological processes occurring within the tumor microenvironment. “It means that you have a tumor that is actively releasing live cells in the body, and because they are whole cells, they not only can be used for DNA analysis, but also for RNA analysis and protein analysis,” she said.
While researchers have largely focused on studying circulating tumor cells found in peripheral blood, they are also found in other bodily fluids, such as pleural and peritoneal effusions and cerebrospinal fluid.2 In the case of bladder cancer, these cells can also be found in urine.
Circulating Tumor Cells Test: Clinical Challenges
A circulating tumor cell assay can identify these cells in a sample of peripheral blood. However, several key obstacles currently prevent the widespread use of circulating tumor cells for cancer diagnosis, prognosis, and treatment monitoring. The first is that these cells are rare—as few as one cell in 107 white blood cells in every 10mL of peripheral blood—making them difficult to isolate. The second is that researchers lack a standardized protocol for isolating and studying these cells. “The reason why CTCs are less used right now in the clinic is not because they provide less information, but because they are more difficult to work with,” Reduzzi remarked.
Circulating tumor cell isolation
To isolate and enrich these rare cells in a circulating tumor cell blood test, researchers often use morphological characteristics, such as size, or biological properties, such as their surface antigens.1 However, Reduzzi said that both of these approaches have limitations, and cannot always isolate all the cells within a sample: “Each technology always will have a bias.” Microfluidics technologies have also been developed for the isolation of these cells.1
Immunofluorescence shows that this circulating tumor cell isolated from peripheral blood is positive for cytokeratin (CK+, green), an epithelial protein used to identify cancer cells.
Carolina Reduzzi, Weill Cornell Medical College
Reduzzi believes that more recent technologies, including high-throughput instruments and advanced imaging, will make circulating tumor cell analysis easier for researchers. “You take all the cells that you find in the blood tube, and you analyze all of them. You do staining, and you try to look for all these cells that are not normal, and you have all these different categories,” said Reduzzi. “I’m very hopeful with these new technologies, that they will be able to show the value of CTCs much quicker.”
Circulating Tumor Cell Analysis and Clinical Applications
Scientists study circulating tumor cells because they reflect the biological and genetic complexity of the tumors from which they arise. They also use circulating tumor cells in cancer diagnosis and prognosis, treatment monitoring, and the development of personalized medicines.
Understanding metastasis
Clinicians can identify cancer patients at high risk of metastasis based on the number of circulating tumor cells in their blood. Yet the biological processes underpinning metastasis are not well understood, and for every 10,000 circulating tumor cells, only one is likely to cause metastasis.2
“It’s actually a very inefficient process, because these cells are released, and they are not in their environment, so most of them will die because of the stress of being in the blood vessel or will be killed by the immune cells that will recognize them,” Reduzzi explained. “And even if they survive, it doesn’t mean that they can form a secondary growth.”
However, researchers have shown in mouse models that circulating tumor cells can also be released as clusters rather than individual cells; these clusters provide shelter that makes some of their residents more likely to survive. “It’s now pretty accepted in the field that CTCs can travel alone or as clusters, and it seems that these clusters are the ones with the highest metastatic potential,” said Reduzzi.
Liquid biopsy and early detection of cancer
By collecting and studying circulating tumor cells from peripheral blood samples—otherwise known as liquid biopsy—scientists can gain a deeper understanding of tumors in a less invasive manner than traditional tumor biopsy methods.
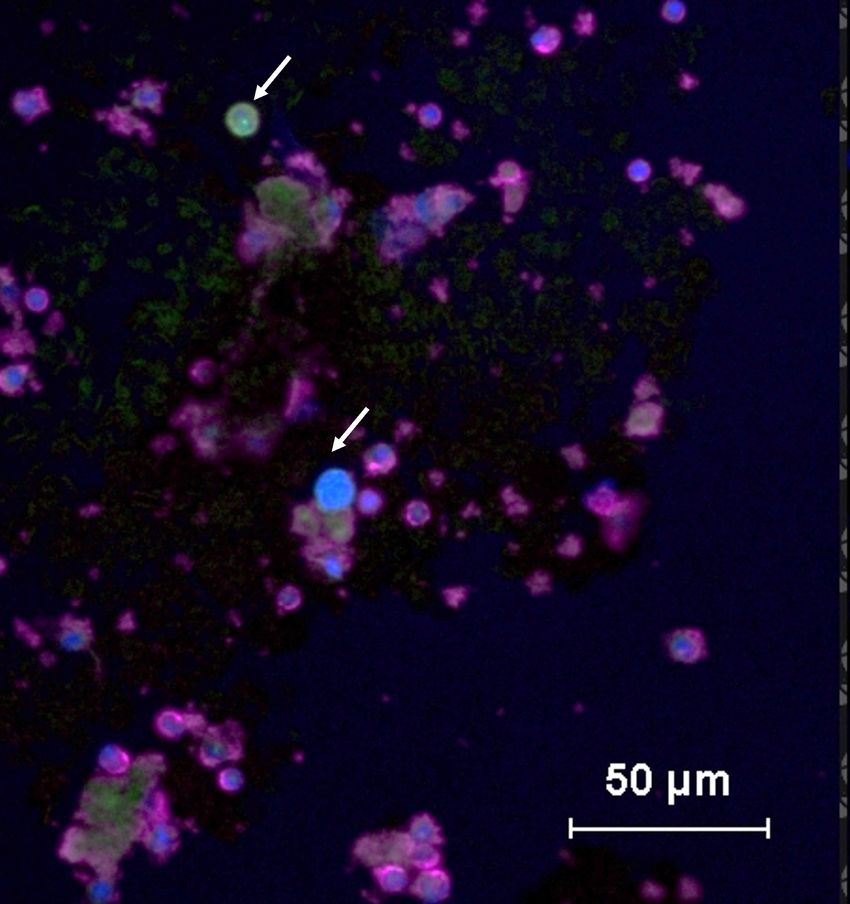
In this immunofluorescence image, circulating tumor cells are positive for cancer-associated protein cytokeratin (CK+, green), while normal peripheral blood mononuclear cells are positive for leukocyte common antigen (CD45+, purple).
Carolina Reduzzi, Weill Cornell Medical College
Circulating tumor cells may be present and detectable in patients whose tumors are not yet large enough to be accurately identified using standard diagnostic imaging methods, such as magnetic resonance imaging.4 Researchers can also use the presence of these cells in the blood as a less invasive method for the early detection of tumors, which is crucial in providing rapid treatment and preventing disease progression.5
Reduzzi is enthusiastic about using these cells for liquid biopsy and serves as the chair of the Young Committee of the International Society of Liquid Biopsy. The committee has an ongoing survey that aims to evaluate the attitudes and practices of oncologists around liquid biopsy and understand the barriers that currently prevent liquid biopsy from being widely used.
Cancer prognosis with circulating tumor cells
Perhaps one of the most dramatic examples of the clinical application of circulating tumor cells is in breast cancer prognosis. Large-scale clinical studies of thousands of metastatic breast cancer patients have shown that those with more than five circulating tumor cells per 7.5 millilitres of blood are much more likely to have a worse prognosis with poorer survival and more aggressive disease.6 This striking finding led researchers to propose a further stratification of metastatic breast cancer patients into Stage IV indolent and Stage IV aggressive.
According to Reduzzi, there was almost a linear relationship between the number of cells above the cutoff of five and poorer clinical outcomes. “In early breast cancer, it’s only one—if you have even one circulating tumor cell already, your prognosis is going to be worse than if you have zero,” she said.
Researchers have shown that the same cutoff also applies to metastatic prostate cancer, while in colorectal cancer, the cutoff is three cells per 7.5mL of blood.7,8 Their use in cancer prognosis has now been demonstrated in the majority of solid tumors. 9
Treatment monitoring with circulating tumor cells
Researchers can measure the levels of circulating tumor cells to monitor a patient’s response to cancer treatment, with a reduction in the numbers of cells throughout treatment linked to better clinical outcomes.
For example, in a study of metastatic colorectal cancer, some patients who had an unfavorable circulating tumor cell profile prior to commencing treatment demonstrated a conversion to a favorable profile after three to five weeks of treatment; this group of patients had significantly longer progression-free and overall survival.8
Personalized medicine
Reduzzi said that clinicians can also provide personalized treatments for individual patients based on the analysis of their circulating tumor cells, which typically express the same surface markers as the tumors that spawn them. For example, patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer can receive HER2 inhibitors. “With CTCs, you could look and monitor over time during the treatment, if that marker is still expressed by your tumor or not,” Reduzzi explained.
Reduzzi is determined to overcome the current technological limitations and see the applications of these cells translated into clinical practice. “If we can really understand circulating tumor cells, how they work, how they survive, and how they grow, that’s the key to understanding the metastatic process and potentially finding a way to stop it,” she commented.
- de Wit S, et al. Detection of circulating tumor cells. Scientifica. 2014;2014:819362.
- Bates M, et al. Circulating tumour cells: the good, the bad and the ugly. Biochim Biophys Acta BBA – Rev Cancer. 2023;1878(2):188863.
- Lin D, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6(1):404.
- Vidlarova M, et al. Recent advances in methods for circulating tumor cell detection. Int J Mol Sci. 2023;24(4):3902.
- Harouaka R, et al. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141(2):209-221.
- Gerratana L, et al. Mapping breast cancer therapy with circulating tumor cells: the expert perspective. The Breast. 2025;81.
- de Bono JS, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer.Clin Cancer Res. 2008;14(19):6302-6309.
- Cohen SJ, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
- Reduzzi C, et al. Unveiling the impact of circulating tumor cells: Two decades of discovery and clinical advancements in solid tumors. Crit Rev Oncol Hematol. 2024;203:104483.