One of Australia’s most iconic species, the koala is staring down the barrel of extinction. Among a barrage of other threats, including deforestation, attacks by wild and domestic dogs, and traffic collisions, koala populations are being decimated by chlamydia. The sexually transmitted bacterial infection affects about 50 percent of koalas across Australia and can be fatal.1
Now, after more than a decade of research, scientists from the University of the Sunshine Coast (UniSC) have received approval for a single-dose chlamydia vaccine that they hope will turn the tide. “This is the first chlamydia vaccine for koalas,” said Sam Phillips, a molecular microbiologist at UniSC who helped develop the vaccine. “We believe it will help prevent koalas from becoming infertile and getting advanced disease.”
Chlamydia Infections Endanger Koalas
Chlamydia pecorum infections—widely prevalent in wild koala populations—have a range of horrific impacts on the animals as the bacteria spread throughout their organs. Infections of the conjunctiva can result in permanent blindness, while urinary tract infections can progress into inflammation of the bladder wall and kidneys and cause the development of abscesses and cysts. In the reproductive tract, the disease is known to cause infertility in females, and at least one study suggests that it can also result in DNA damage to the sperm of male koalas.2
Molecular microbiologist Sam Phillips, of UniSC, helped develop a vaccine that targets the major outer membrane protein of Chlamydia pecorum.
UniSC
“When [koalas] get ocular disease, they can’t find leaves to eat, they can’t find the trees to climb, and they can’t run away from predators,” explained Phillips. “Then the urinary tract infections lead to dehydration and muscle wasting. They can’t climb trees to get away from predators.” These effects have caused massive declines in koala populations across much of the country.
“Chlamydia has been around in koalas for a long time, and it hasn’t always been an issue. But what we like to say is that when there were millions of koalas around, the populations could deal with these ebbs and flows of chlamydial disease,” Phillips observed. But the small size, extreme isolation, and often limited genetic diversity of current koala populations mean that the sleepy marsupials have far less resilience to disease.
Until now, treatment for C. pecorum infections has been limited to antibiotics. Unfortunately, Phillips explained, the koala genome has major expansions to its cytochrome p450 gene family that enable koalas to detoxify chemicals and drugs.3 “Koalas need to have four times the dose of what you would give an animal of similar size, just because they process the antibiotics so quickly,” he said.
Although effective, this high dose of antibiotics strips the koalas’ gastrointestinal tract of highly specialized microbiota that allow them to detoxify and digest their sole diet of eucalyptus leaves, resulting in starvation in some treated individuals. These drawbacks have been major drivers for the development of a vaccine. “We hope that we can vaccinate healthy koalas, and they’re less likely to need to come in and get antibiotics,” Phillips added.
Why Developing a Chlamydia Vaccine for Koalas Is Challenging
Developing a vaccine for C. pecorum has been no small feat, and the team at UniSC faced several major bottlenecks in the development pipeline. The vaccine is based on the major outer membrane protein (MOMP) of the bacteria; by targeting conserved regions of MOMP across three dominant genotypes, the treatment offers broad protection.4 However, MOMP is a double-edged sword. “It has some highly hydrophobic regions, which make it fairly stable but difficult to produce. We still can’t produce really high yields.” Phillips remarked.
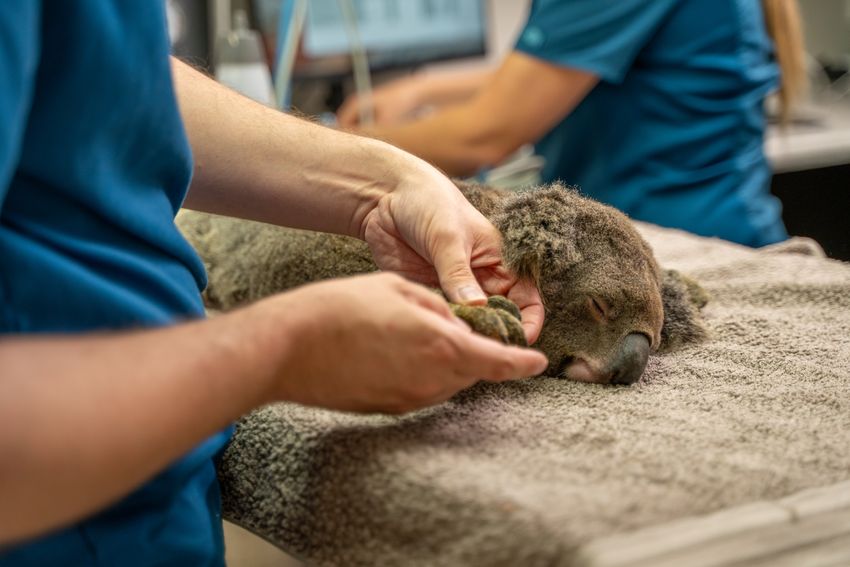
Koalas that are admitted to wildlife hospitals will receive the single-dose vaccine, which researchers hope will reduce the need for antibiotics.
UniSC
Complicating the situation further, C. pecorum is a biphasic bacterium: It has both intracellular and extracellular phases of its life cycle. To target both phases, Phillips and his colleagues included several adjuvants in the vaccine. “One is poly I:C, which mimics intracellular infections, and then we use a peptide which is a mimic of a host defense peptide, which traditionally targets extracellular antigens,” Phillips said.
Delivery methods provided another challenge for the team. “The third component is a carrier molecule, so it forms a capsule around the antigen and the adjuvants, which is biodegradable,” said Phillips, noting that instead of getting just a response in the arm, the carrier molecule allows for dissemination across the body, and then it degrades.
Unfortunately, like the adjuvants and MOMP, the carrier molecule is also difficult and expensive to make. “We’ve partnered with some pharmaceutical companies to get access to some of these newer, next-generation adjuvants to try and improve our responses and lower dose costs,” Phillips commented.
Historic Vaccine Approval Could Save Koalas from Chlamydia Deaths
Unlike previous attempts, the current vaccine is a single-dose treatment, meaning that koalas won’t need to be kept in captivity for long periods between doses. The recent minor-use approval of the vaccine by the Australian Pesticides and Veterinary Medicines Authority is based on a decade of clinical data that demonstrated its effectiveness: Treated koalas had lower rates of chlamydia and a 64 percent reduction in disease-related mortality.

The vaccine contains multiple components, including conserved regions of the MOMP protein, two key adjuvants, and a carrier molecule that biodegrades after about 24 hours.
UniSC
Outside of UniSC, the study was made possible by multidisciplinary collaborators, including pharmaceutical partners, wildlife hospitals, veterinarians, ecologists, the RSPCA and other wildlife funds, and the Australian government. Phillips recalled that it was a long, hard slog for the team. “There have been times where we thought we were going to fail. There have been hurdles that we could get over, and very slowly, we’ve pushed through and got to this point.”
The two-year minor use approval means that the vaccine can be rolled out in wildlife hospitals and veterinary clinics across regions of the country that are home to the most affected populations. “We have funding to produce 500 doses that should be available early next year,” said Phillips. However, the team has already received more than 500 requests for doses, and they will need more funding to continue the rollout. “At the moment, we’re really hoping that we get some more funds so that we can make more vaccines available,” Phillips added.
Phillips said the approval is a major success but emphasized that the vaccine is not a silver bullet that will eliminate chlamydia infections across the continent. Rather, the treatment should be used alongside other key conservation measures, including reducing deforestation. “We should be able to protect these populations from continuing decline and be able to get a positive population growth,” Phillips concluded.

