In the 1960s, psychedelic drug research was reportedly on the brink of a breakthrough, deemed a silver bullet that could treat a plethora of psychiatric disorders. Researchers found that drugs such as psilocybin (the primary psychoactive constituent in “magic mushrooms”) and lysergic acid diethylamide (LSD or “acid”) could save one from the crippling grips of depression, addiction, and even sexual dysfunction with just a single dose.1 However, the United States government perceived psychedelics as a destabilizing threat to individuals and society, and in 1970, the Controlled Substances Act quashed research on psychedelics by categorizing them as Schedule I: drugs with high abuse potential and no medical use.
The past two decades have witnessed a “Psychedelic Renaissance,” where enthusiastic users and investors claim that these drugs can pack years of therapy into a few discrete high-dose experiences. There has been some evidence to back these claims. For instance, in the largest psychedelics trials to date with more than 100 patients, researchers have found significant improvements in symptom severity in several psychiatric conditions. One study found that a single high dose of psilocybin administered to patients with treatment-resistant depression, in combination with psychological support, produced larger reductions in depression symptoms for up to three weeks compared to moderate and very low doses.2 Likewise, two studies in patients with posttraumatic stress disorder (PTSD) found larger reductions in PTSD symptoms up to eight weeks following three doses of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy” or “molly”) compared to an inactive placebo.3,4 Other work has shown promising initial results for the treatment of addiction, generalized anxiety disorder, obsessive-compulsive disorder, and anorexia.5–8 In fact, the FDA granted Breakthrough Therapy designation to MDMA, psilocybin, and LSD to expedite drug development.
In parallel with clinical trials, basic research and animal studies have aimed to illuminate fundamental mechanisms of psychedelic drug effects. Although clear effects at molecular and cellular levels of analysis have been reported, how psychedelics might produce beautiful visuals, profound belief change, a heightened sense of knowledge, and whether such effects are even necessary for their therapeutic efficacy is only beginning to be clarified.9–11 Although some have claimed that these drugs are “microscopes” of the mind that could help illuminate how the mind and brain operate, such unbridled enthusiasm can make it seem like the researchers themselves “drank the Kool-Aid”.12–15 It is therefore imperative that these drugs be understood through a sober lens. Let’s start with the basics.
What are Psychedelics?
The term psychedelic, coined by the psychiatrist Humphry Osmond, conjoined the Greek words “psyche” and “delos” to mean “mind manifesting.” Psychedelics can loosely refer to a range of pharmacologically distinct hallucinogens, but here, we limit the definition to those drugs most commonly described as psychedelics: hallucinogenic serotonin 2A (5-HT2A) agonists, such as psilocybin and LSD. Other common psychedelics under this category include mescaline, found in peyote and San Pedro cacti, and N,N-dimethyltryptamine (DMT), the major psychoactive constituent in the Amazonian brew ayahuasca. MDMA is sometimes classified as an empathogen or entactogen, which is a separate class from psychedelics, as it produces less drastic hallucinogenic effects, and its R-enantiomer only weakly binds to 5-HT2A receptors. However, coadministration with a 5-HT2A antagonist diminishes MDMA’s subjective and cognitive effects, suggesting that it may share certain mechanisms with prototypical psychedelics.16,17 While researchers believe MDMA’s facilitation of social bonding and feelings of empathy to be largely due to its release of norepinephrine, dopamine, and, especially, serotonin, other psychostimulants with similar pharmacology such as amphetamine fail to produce these entactogenic effects.
Psychedelics’ Mechanisms of Action on the Brain at a Molecular Level
At the level of molecular pharmacology, there is reasonable certainty of the importance of the serotonin pathways in the action of psychedelics. This is perhaps unsurprising, given that the disorders being investigated with psychedelic treatments are commonly treated with FDA-approved medications that impact serotonin pathways. Psilocybin, LSD, mescaline, DMT, and more obscure psychedelics with similar subjective effects are all agonists at the 5-HT2A receptor, and the chemical structures of many psychedelics resemble serotonin. Although other serotonin receptor subtypes (e.g., 5-HT1A and 5-HT2C) may play a non-negligible role in the action of different psychedelics, the affinity of psychedelics for 5-HT2A receptors is tightly linked to their potency.18 In fact, blocking 5-HT2A receptors prevents the bulk of subjective effects and can even abort a “trip” that has already begun.19,20
The structure of serotonin (left) closely mirrors that of psilocybin (right), which rapidly metabolizes to psilocin and activates various serotonin receptors, with activity at serotonin 2A (5-HT) receptors largely promoting psychedelic effects.
Manoj Doss (MolView), adapted by Erin Lemieux
Cellular neuroscientists have recently promoted a hypothesis that psychedelics enhance neural plasticity, which refers to various neural changes, particularly those that occur at synapses. Researchers think plasticity underlies various forms of learning and memory and is impaired across a range of psychiatric disorders.21 The primary evidence for psychedelic-induced plasticity comes from enhancements of dendritogenesis (growth of dendrites) and spinogenesis (growth of spines, which are protrusions on dendrites that are the specific site of synapses). A single dose of a psychedelic has been reported to increase the number of dendrites and spines in animal models.22 These effects can occur within hours of dosing, increase over the next several days, and can be maintained for up to a month.23
Psychedelics may facilitate other forms of plasticity as well such as the induction of long-term potentiation, the strengthening of the influence one neuron has upon another.24,25 Although psychedelics reliably induce multiple markers of plasticity in the cortex on pyramidal cells (the main excitatory neurons), where 5-HT2A receptors are densely distributed, such enhancements are less consistent or are even observed as reductions in the hippocampus, where 5-HT2A expression is lower and largely limited to inhibitory interneurons.26–28 Likewise, some scientists reported psychedelics to increase neurogenesis (growth of new neurons) in the hippocampus while others have found contrary results.27
Even if psychedelics increase certain forms of plasticity, greater plasticity is not necessarily beneficial. Psychostimulants, such as amphetamine, also increase structural synaptic plasticity, though these effects may require multiple doses and involve other regions such as the striatum.29 Some researchers have found that plasticity induction in the striatum and hippocampus, brain structures associated with reward and spatial learning, respectively, may require specific environmental input during “critical periods” in the days following psychedelic administration to effect changes to these structures and the behaviors they support.30,31 While so far, researchers haven’t found psychedelics to open human-specific critical periods such as language learning, the psychedelic experience is anecdotally described as making one “child-like” again. However, the weight-based doses in animal models that promote plasticity are an order of magnitude larger than those typically ingested by humans, rendering the generalizability of these findings questionable.
A Systems and Cognitive Neuroscience Models Approach to Understand the Psychedelic Mechanism
It remains unclear how 5-HT2A activation results in the distortions in perception produced by psychedelics. Moreover, many measures of neural plasticity cannot be readily measured in humans, obfuscating the relationship between enhanced plasticity and mental health improvements. To understand such high-level phenomena, researchers have taken a systems- and cognitive-level approach to interrogate brain function. Often, they use functional magnetic resonance imaging (fMRI), which measures local changes in blood oxygenation (a proxy to neural activity), to investigate the acute and post-acute effects of psychedelics on human brain circuits and networks. However, many of these effects fail to replicate, particularly post-acute drug effects.32 Thus, we limit much of this discussion to acute effects.
Thalamic Gating Model
The thalamic gating model, an early circuit model of psychedelic drug action, proposed that psychedelics attenuate sensory filtering by the thalamus.33 By activating 5-HT2A-expressing excitatory neurons in the cortex, these neurons activate inhibitory neurons in the striatum that inhibit inhibitory neurons of the globus pallidus, thereby releasing the thalamus from inhibition. Neuroscientists theorize that this thalamic “disinhibition” inundates the cortex with sensory information and creates a positive feedback loop that can lead to perceptual distortions, heightened distractibility, and an overwhelming loss of perceived control. Several fMRI studies have found that psychedelics increase the coupling of brain activity (i.e., “functional connectivity”) between the thalamus and the cortex under task-free (i.e., “resting-state”) conditions.34–37 Moreover, research in animals and humans suggests that sensory filtering under acute psychedelic effects is impaired. During the acute effects of psychedelics, rodent auditory neurons fail to attenuate their responses to familiar stimuli, and both human and animal work have found reductions in prepulse inhibition, a sensory filtering process that occurs when a startle response to a loud tone is attenuated when preceded by a softer tone.38–40 However, psychedelics may have no impact or even enhance prepulse inhibition when the delay between the two tones is larger.41,42 It is also unclear how the thalamic gating model relates to the persisting therapeutic effects of psychedelics.
A Popular but Problematically Unfalsifiable Model: REBUS
Another model that has gained traction in recent years is RElaxed Beliefs Under pSychedelics (REBUS).43 REBUS focuses on the default mode network (DMN), a canonical large-scale cortical network that supports a range of cognitive functions, including social processing, self-related processing, episodic memory, and semantic memory.44 Notably active when an individual is in a resting-state condition, REBUS postulates that the DMN sits at the top of the brain’s information processing hierarchy and that psychedelics disrupt this hierarchy by attenuating DMN function. Several studies have found psychedelics to attenuate (resting-state) functional connectivity among regions of the DMN.45–50 Given the DMN’s role in high-level belief representations (i.e., semantic memories not necessarily shared by others), scientists think that the effects of psychedelics on DMN functional connectivity relax one’s assumptions about the world, thereby leading to more uncertainty and perceived novelty. Due to the DMN’s role in self-related processing, researchers hypothesize that attenuation of DMN function produces “ego dissolution,” a variable effect of psychedelics in which one’s sense of self is temporarily lost. Finally, by disrupting the top of the brain’s hierarchy, which supposedly constrains the activity of lower levels, they think neural activity could become more complex, as indexed by measures of entropy and signal compressibility, leading to a wider plethora of brain states, including less communication within brain networks and greater communication between networks. These effects are thought to make one more sensitive to low-level sensory information from the environment that could allow for the revision of maladaptive beliefs. For example, after years of suffering from depression or PTSD, one may hold maladaptive beliefs that they are not a good person or that the world is dangerous. “Unlearning” such beliefs may therefore provide therapeutic benefits.
A major problem with REBUS is its lack of falsifiability. An idea is falsifiable if it is possible to conceive of evidence that can prove the idea wrong. If a theory can evade any fathomable contradiction, it is typically considered outside the realm of science, or at least that which is worth testing. REBUS is largely based on the free energy principle and bears similarities to a controversial theory of consciousness, integrated information theory.51,52 Both the free energy principle and integrated information theory are considered to be unfalsifiable, and thus, certain tenants of REBUS are also largely unfalsifiable.53,54
There are also more specific issues with which REBUS must grapple. First, psychedelics can paradoxically produce a sense of familiarity and strengthen beliefs, including the potential for false beliefs.10,55 Second, the specificity of the DMN in mediating psychedelic effects is questionable. While one study found that attenuation of DMN functional connectivity was associated with ego dissolution, a replication attempt with higher quality data found a near-zero correlation.35,46 Moreover, other drugs have been found to acutely attenuate DMN functional connectivity, including alcohol, cannabis, coffee, amphetamine, and even a single non-psychoactive dose of the antidepressant sertraline.56–60 In contrast, ketamine, a pharmacologically distinct dissociative drug frequently compared to psychedelics and widely used for treating depression (largely off-label, though its S-enantiomer is FDA-approved), has been found to decrease, have no effect, or even increase functional connectivity within the DMN.61–66 Post-acute effects of psychedelics on DMN connectivity are also largely inconsistent, with some studies finding no effects, increases, decreases, or both in the same dataset, depending on the analysis method.49,67–73 Studies with more than 500 participants have also found both increased and decreased DMN functional connectivity in depression, questioning this metric as a useful biomarker for depression.74,75
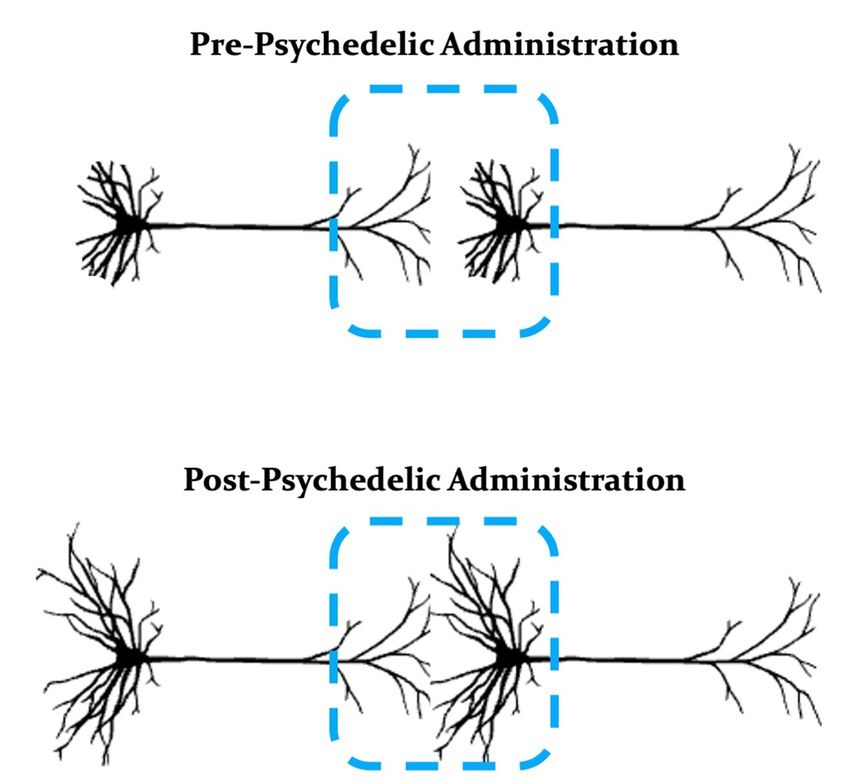
Example neurons (pyramidal cells) with protracted dendrites prior to the administration of a psychedelic (top). Following administration of a psychedelic, the dendrites on pyramidal cells may grow, thereby leading to new connections between neurons (bottom).
Manoj Doss, PhD, adapted from https://bioicons.com/
While it may be tempting to infer that increased neural complexity produced by psychedelics is related to increased synaptic plasticity, neural complexity increases are most stark during acute drug effects and inconsistently found post-acutely. In contrast, plasticity increases become more prominent post-acutely. These neural complexity increases may also not necessarily index unique cognitive operations promoted by psychedelics but may rather be an artifact of the use of resting-state neuroimaging. When one is asked to rest in a scanner under drug-free conditions, the array of cognitive operations is likely to be minimal and focused primarily on internal mentation such as daydreaming. Moreover, more than 40 percent of participants fall asleep under (drug-free) resting-state conditions, and sleep is associated with less neural complexity.76,77 Conditions that promote internal mentation also promote DMN activity, hence the high level of DMN engagement observed at rest, and deploying attention to the environment can attenuate DMN engagement. The acute effects of psychedelics preclude sleep, thereby promoting a broader array of cognitive operations focused on both external attention and internal mentation, such as shifting attention to scanner noises, opening and closing one’s eyes, and thinking about future plans. However, none of these cognitive operations are unique to the psychedelic experience. Therefore, simply having participants under drug-free conditions perform a range of cognitive operations should also increase neural complexity. Indeed, two studies found that having participants perform complex cognitive operations (i.e., a perceptual task or watching a movie) under drug-free conditions not only increased neural complexity compared to resting-state, but this increase in complexity reached levels similar to those produced by psychedelics under resting-state conditions or when performing the same cognitive operations.49,78 Engaging in relatively boring tasks under psychedelics might even be expected to attenuate neural complexity by removing one from a psychedelic experience (known as “grounding”). However, performing these tasks under psychedelics also tended to increase neural complexity. If neural complexity were indexing unique psychedelic effects, engaging in task-directed cognitive operations under drug-free conditions should never reach psychedelic levels of neural complexity. An overarching systems-level model of psychedelic drug action should be able to capture psychedelic effects across conditions and not be so fragile to the introduction of task manipulations.
So New yet So Familiar: Psychedelics, Memory, and Belief Change
Instead of an overarching model of psychedelic drug action, our group has recently focused on the influence of psychedelics on different learning and memory systems, especially given that plasticity is thought to support the acquisition and updating of memories. What one takes away from a psychedelic experience, that is, a memory, may have great explanatory value for the persisting effects of these drugs on mental health. For example, several studies have found that the acute and post-acute effects of psychedelics can enhance extinction learning of conditioned fear.79 Extinction learning occurs when a stimulus (e.g., a sound) previously associated with an aversive outcome (e.g., a shock) is presented several times without the aversive outcome, leading to new learning that the stimulus no longer portends threat. Extinction learning is thought to be a useful experimental model of behavioral therapies, such as exposure therapy for PTSD in which patients repeatedly confront the trauma memory to reduce symptoms. Unfortunately, fear can readily return following extinction, and thus, enhancing extinction learning using psychedelics may improve the efficacy of exposure-based treatments.
Psychedelics do not enhance all learning processes and can even impair certain forms of learning, though such differential effects may not necessarily be deleterious. A common conjecture is that psychedelics enhance cognitive flexibility, or the capacity to switch between cognitive operations in the face of a changing environment. In contrast, the acute and post-acute effects of psychedelics have been found to both impair and enhance measures of cognitive flexibility, potentially explained by differential effects on learning processes.67,80–83 One of the more striking findings is how psychedelics differentially affect the formation of memories supported by the hippocampus versus the cortex. Like psychoactive drugs that promote inhibition or impairments in long-term potentiation such as alcohol, ketamine, and cannabis, the acute effects of psychedelics reliably impair the formation of hippocampal-dependent episodic memories, consistent with the prominent 5-HT2A expression on inhibitory neurons in the hippocampus.84 In contrast to these other drugs and consistent with 5-HT2A-expressing excitatory neurons in the cortex, we found in three separate studies that psychedelics uniquely spared or even enhanced the formation of a cortical-dependent memory called familiarity.84,85 Familiarity occurs when one knows that a stimulus was recently experienced without necessarily recalling specific details such as recognizing a face without remembering from where or when one met this individual. One explanation is that psychedelics could initially heighten the perceived novelty and subsequent information processing of any given stimulus, but greater information processing promotes greater subsequent familiarity. Such an account could explain the paradoxical heightening of both novelty and familiarity produced by psychedelics. This cortically dominant mode of information processing under acute psychedelic effects coupled with post-acute enhancements of cortical plasticity could fast-track the instantiation of new semantic memories, which typically form over months to years, thereby disrupting crystallized maladaptive beliefs. Indeed, artificially enhancing cortical plasticity can disrupt semantic-like memories in mice.86 However, heightened familiarity can also drive perceived, but illusory, truth, a phenomena known as the illusory truth effect.87 If this hypothesis is correct, we must focus on how to displace specific maladaptive beliefs while avoiding the induction of false beliefs.
The Simple Truth of Complexity in Psychedelic Research
The clinical trials testing the therapeutic efficacy of psychedelics have largely followed a standard psychological support protocol with preparation sessions in which patients build rapport with therapists, a dosing session in which participants ingest a drug and lie on a couch while wearing eyeshades and listening to music, and integration sessions in which participants discuss with therapists their dosing session experience. This psychological support model has, until recently, been thought to be integral to the therapeutic effects of “psychedelic-assisted therapy.” However, the psychological support embedded in these trials has not been properly standardized, shown to be efficacious as a stand-alone treatment, or ever empirically tested as a key contributor to the therapeutic effects of psychedelic administration.88,89 This leads one to question whether this “couch method” is the best practice in dosing sessions to drive maximal therapeutic benefit.79 Moreover, there has been, in some studies, an underemphasis on characterizing the rate and severity of adverse events, including the higher rates of suicidal ideation observed in the active treatment groups. These and other limitations may have contributed to the FDA’s rejection of MDMA-assisted therapy for the treatment of PTSD, the first psychedelic therapy package to reach the New Drug Application stage, despite the impressive efficacy data. An understanding of the basic mechanisms underlying the acute and post-acute effects of psychedelics could help delineate the conditions or types of psychotherapy that maximize the benefits of these drugs and minimize their harms.
In summary, we must continue to be cautious of the ongoing hype of psychedelics stirred by popular media, both the panacea-like nature of these drugs and propaganda campaigns of overstated harms. We must also be wary of overly simplistic explanations for how psychedelics work that largely emphasize such biases. In fact, drugs that may treat a broad array of psychiatric disorders, that relax and strengthen beliefs, that produce a sense of novelty and familiarity, that impair and enhance cognitive flexibility, and that impair and enhance memory are unlikely to have simple explanations.
Disclosures: M.K.D. is an advisor to VCENNA. G.A.F. has served as a consultant for SynapseBio AI, and he is a stockholder in Alto Neuroscience. C.B.N. is a consultant for Engrail Therapeutics, Clexio Biosciences LTD, Sero (previously Galen Mental Health LLC), Goodcap Pharmaceuticals, Sage Therapeutics, Senseye Inc, Precisement Health, Autobahn Therapeutics Inc, EMA Wellness, Denovo Biopharma LLC, Alvogen, Acadia Pharmaceuticals, Inc, Reunion Neuroscience, Kivira Health, Inc, Wave Neuroscience, Patient Square Capital LP, Invisalert Solutions Inc., and Neurocrine Biosciences, LLC. C.B.N. owns the following patents: Method and devices for transdermal delivery of lithium (US 6,375,990B1), Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2), Compounds, Compositions, Methods of Synthesis, and Methods of Treatment (CRF Receptor Binding Ligand) (US 8,551, 996 B2). C.B.N. owns stock in Corcept Therapeutics Company, EMA Wellness, Precisement Health, Signant Health, Galen Mental Health LLC, Kivira Health, Inc., Denovo Biopharma LLC, and Senseye Inc. None of these groups had any role in the writing of this article.
- Rucker JJH, et al. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology. 2018;142:200-218.
- Goodwin GM, et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N Engl J Med. 2022;387(18):1637-1648.
- Mitchell JM, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033.
- Mitchell JM, et al. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat Med. 2023;29(10):2473-2480.
- Bogenschutz MP, et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients With Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2022;79(10):953.
- MindMed Receives FDA Breakthrough Therapy Designation and Announces Positive 12-Week Durability Data From Phase 2B Study of MM120 for Generalized Anxiety Disorder. March 7, 2024. Accessed December 18, 2024.
- Moreno FA, et al. Safety, Tolerability, and Efficacy of Psilocybin in 9 Patients With Obsessive-Compulsive Disorder. J Clin Psychiatry. 2006;67(11):1735-1740.
- Peck SK, et al. Psilocybin therapy for females with anorexia nervosa: a phase 1, open-label feasibility study. Nat Med. 2023;29(8):1947-1953.
- Barksdale BR, et al. The mechanistic divide in psychedelic neuroscience: An unbridgeable gap? Neurotherapeutics. 2024;21(2):e00322.
- McGovern HT, et al. An Integrated theory of false insights and beliefs under psychedelics. Commun Psychol. 2024;2(1):69.
- Olson DE. The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol Transl Sci. 2021;4(2):563-567.
- Grof S. LSD Psychotherapy. Hunter House; 1980.
- Yaden DB, et al. Psychedelics and Consciousness: Distinctions, Demarcations, and Opportunities. International Journal of Neuropsychopharmacology. 2021;24(8):615-623.
- Aday JS, et al. Personal Psychedelic Use Is Common Among a Sample of Psychedelic Therapists: Implications for Research and Practice. Psychedelic Medicine. 2023;1(1):27-37.
- Jylkkä J and Mustamo A. Psychedelic researchers’ own experiences of psychedelic substances, their link to opinions of psychedelics, and reflections on positionality. Psychopharmacology. Published online August 11, 2025.
- Liechti M. Psychological and Physiological Effects of MDMA (“Ecstasy”) after Pretreatment with the 5-HT2 Antagonist Ketanserin in Healthy Humans. Neuropsychopharmacology. 2000;23(4):396-404.
- van Wel JHP, et al. Blockade of 5-HT2 Receptor Selectively Prevents MDMA-Induced Verbal Memory Impairment. Neuropsychopharmacology. 2011;36(9):1932-1939.
- Glennon RA, et al. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sciences. 1984;35(25):2505-2511.
- Vollenweider FX, et al. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action: NeuroReport. 1998;9(17):3897-3902.
- Becker AM, et al. Ketanserin Reverses the Acute Response to LSD in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Participants. International Journal of Neuropsychopharmacology. 2023;26(2):97-106.
- Appelbaum LG, et al. Synaptic plasticity and mental health: methods, challenges and opportunities. Neuropsychopharmacol. 2023;48(1):113-120.
- Ly C, et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Reports. 2018;23(11):3170-3182.
- Liao C, et al. Structural neural plasticity evoked by rapid-acting antidepressant interventions. Nat Rev Neurosci. 2025;26:101-114.
- de la Fuente Revenga M, et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Reports. 2021;37(3):109836.
- Hesselgrave N, et al. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci USA. 2021;118(17):e2022489118.
- Beliveau V, et al. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J Neurosci. 2017;37(1):120-128.
- Calder AE and Hasler G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacol. 2023;48(1):104-112.
- Tiwari P, et al. Ventral hippocampal parvalbumin interneurons gate the acute anxiolytic action of the serotonergic psychedelic DOI. Neuron. 2024;112(22):3697-3714.e6.
- Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in Neurosciences. 2010;33(6):267-276.
- Nardou R, et al. Psychedelics reopen the social reward learning critical period. Nature. 2023;618(7966):790-798.
- Abad S, et al. MDMA enhances hippocampal-dependent learning and memory under restrictive conditions, and modifies hippocampal spine density. Psychopharmacology. 2014;231(5):863-874.
- Doss MK, et al. Skepticism about Recent Evidence That Psilocybin “Liberates” Depressed Minds. ACS Chem Neurosci. 2022;13(17):2540-2543.
- Vollenweider FX and Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Research Bulletin. 2001;56(5):495-507.
- Tagliazucchi E, et al. Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Current Biology. 2016;26(8):1043-1050.
- Müller F, et al. Altered network hub connectivity after acute LSD administration. NeuroImage: Clinical. 2018;18:694-701.
- Preller KH, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife. 2018;7.
- Avram M, et al. Large-scale brain connectivity changes following the administration of lysergic acid diethylamide, d-amphetamine, and 3,4-methylenedioxyamphetamine. Mol Psychiatry. 2024;30:1297-1307.
- Lane CP, et al. Psilocybin prevents habituation to familiar stimuli and preserves sensitivity to sound following repeated stimulation in mouse primary auditory cortex. bioRxiv. 2024.
- Geyer MA, et al. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156(2-3):117-154.
- Vollenweider FX, et al. The Effects of the Preferential 5-HT2A Agonist Psilocybin on Prepulse Inhibition of Startle in Healthy Human Volunteers Depend on Interstimulus Interval. Neuropsychopharmacol. 2007;32(9):1876-1887.
- Heekeren K, et al. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N,N-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol. 2007;21(3):312-320.
- Gouzoulis-Mayfrank E, et al. Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans: Behavioural Pharmacology. 1998;9(7):561-566.
- Carhart-Harris RL and Friston KJ. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Barker EL, ed. Pharmacological Reviews. 2019;71(3):316-344.
- Menon V. 20 years of the default mode network: A review and synthesis. Neuron. 2023;111(16):2469-2487.
- Carhart-Harris RL, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proceedings of the National Academy of Sciences. 2012;109(6):2138-2143.
- Carhart-Harris RL, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proceedings of the National Academy of Sciences. 2016;113(17):4853-4858.
- Mason NL, et al. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology. 2020;45:2003-2011.
- Palhano-Fontes F, et al. The Psychedelic State Induced by Ayahuasca Modulates the Activity and Connectivity of the Default Mode Network. Hu D, ed. PLOS ONE. 2015;10(2):e0118143.
- Siegel JS, et al. Psilocybin desynchronizes the human brain. Nature. 2024;632(8023):131-138.
- Timmermann C, et al. Human brain effects of DMT assessed via EEG-fMRI. Proc Natl Acad Sci USA. 2023;120(13):e2218949120.
- Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11(2):127-138.
- Tononi G, et al. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci. 2016;17(7):450-461.
- Sun Z, Firestone C. The Dark Room Problem. Trends in Cognitive Sciences. 2020;24(5):346-348.
- IIT-Concerned, et al. The Integrated Information Theory of Consciousness as Pseudoscience. 2023.
- Lawrence DW, et al. N, N-Dimethyltryptamine (DMT)-Occasioned Familiarity and the Sense of Familiarity Questionnaire (SOF-Q). Journal of Psychoactive Drugs. 2023;56(4):443-455.
- Weber AM, et al. A preliminary study on the effects of acute ethanol ingestion on default mode network and temporal fractal properties of the brain. Magnetic Resonance Materials in Physics, Biology and Medicine. 2014;27(4):291-301.
- Wall MB, et al. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. Journal of Psychopharmacology. 2019;33(7):822-830.
- Picó-Pérez M, et al. Coffee consumption decreases the connectivity of the posterior Default Mode Network (DMN) at rest. Front Behav Neurosci. 2023;17:1176382.
- Schrantee A, et al. Effects of dexamphetamine-induced dopamine release on resting-state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging and Behavior. 2016;10(2):548-558.
- Klaassens BL, et al. Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. NeuroImage. 2015;122:440-450.
- Bonhomme V, et al. Resting-state Network-specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers: Anesthesiology. 2016;125(5):873-888.
- Scheidegger M, et al. Ketamine Decreases Resting State Functional Network Connectivity in Healthy Subjects: Implications for Antidepressant Drug Action. Sensi SL, ed. PLoS ONE. 2012;7(9):e44799.
- Zacharias N, et al. Ketamine effects on default mode network activity and vigilance: A randomized, placebo‐controlled crossover simultaneous fMRI/EEG study. Hum Brain Mapp. 2020;41(1):107-119.
- Mueller F, et al. Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network. NeuroImage: Clinical. 2018;19:745-757.
- Niesters M, et al. Effect of Subanesthetic Ketamine on Intrinsic Functional Brain Connectivity: A Placebo-controlled Functional Magnetic Resonance Imaging Study in Healthy Male Volunteers. Anesthesiology. 2012;117(4):868-877.
- Fleming LM, et al. A multicenter study of ketamine effects on functional connectivity: Large scale network relationships, hubs and symptom mechanisms. NeuroImage: Clinical. 2019;22:101739.
- Doss MK, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry. 2021;11(1):574.
- Barrett FS, et al. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Scientific Reports. 2020;10(1).
- Daws RE, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28:844-851.
- Sampedro F, et al. Assessing the Psychedelic “After-Glow” in Ayahuasca Users: Post-Acute Neurometabolic and Functional Connectivity Changes Are Associated with Enhanced Mindfulness Capacities. International Journal of Neuropsychopharmacology. 2017;20(9):698-711.
- Carhart-Harris RL, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Scientific Reports. 2017;7(1).
- Smigielski L, et al. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. NeuroImage. 2019;196:207-215.
- Pasquini L, et al. Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J Psychopharmacol. 2020;34(6):623-635.
- Kaiser RH, et al. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72(6):603.
- Yan CG, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proceedings of the National Academy of Sciences. 2019;116(18):9078-9083.
- Soehner AM, et al. Unstable wakefulness during resting-state fMRI and its associations with network connectivity and affective psychopathology in young adults. Journal of Affective Disorders. 2019;258:125-132.
- Burioka N, et al. Approximate Entropy in the Electroencephalogram during Wake and Sleep. Clinical EEG and Neuroscience. 2005;36(1):21-24.
- Mediano PAM, et al. Effects of External Stimulation on Psychedelic State Neurodynamics. ACS Chem Neurosci. 2024;15(3):462-471.
- Doss MK, et al. How Psychedelics Modulate Multiple Memory Mechanisms in Posttraumatic Stress Disorder. Drugs. 2024;84:1419-1443.
- Pokorny T, et al. LSD acutely impairs working memory, executive functions, and cognitive flexibility, but not risk-based decision-making. Psychological Medicine. 2020;50(13):2255-2264.
- Wießner I, et al. LSD, afterglow and hangover: Increased episodic memory and verbal fluency, decreased cognitive flexibility. European Neuropsychopharmacology. 2022;58:7-19.
- Kanen JW, et al. Effect of lysergic acid diethylamide (LSD) on reinforcement learning in humans. Psychol Med. 2023;53(14):6434-6445.
- Egner T. Principles of cognitive control over task focus and task switching. Nat Rev Psychol. 2023;2(11):702-714.
- Doss MK, et al. Unique effects of sedatives, dissociatives, psychedelics, stimulants, and cannabinoids on episodic memory: A review and reanalysis of acute drug effects on recollection, familiarity, and metamemory. Psychological Review. 2024;131(2):523-562.
- Doss MK, et al. Psilocybin and 4-Bromo-2,5-Dimethoxyphenethylamine (2C-B) at Encoding Distort Episodic Familiarity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2024;9(10):1048-1057.
- Navarro Lobato I, et al. Increased cortical plasticity leads to memory interference and enhanced hippocampal-cortical interactions. eLife. 2023;12:e84911.
- Dechêne A, et al. The Truth About the Truth: A Meta-Analytic Review of the Truth Effect. Pers Soc Psychol Rev. 2010;14(2):238-257.
- Goodwin GM, et al. Must Psilocybin Always “Assist Psychotherapy”? AJP. 2024;181(1):20-25.
- Hultgren J, et al. A dose of therapy with psilocybin – A meta-analysis of the relationship between the amount of therapy hours and treatment outcomes in psychedelic-assisted therapy. General Hospital Psychiatry. 2025;96:234-243.

