Chronic kidney disease (CDK) is pervasive within the global population, affecting over 700 million people. The condition is widespread in both number of individuals affected and in causes. Existing medical conditions, environmental factors, and genetics all play a role in the development of CDK in an individual.
One of the known genetic risk factors include a mutation in the apolipoprotein L1 (APOL1) gene, with two known risk variants dubbed G1 and G2. While these variants are rare in most populations, they have a higher prevalence in people with West African ancestry with up to 13% of the population having two variants, and up to 38% being carriers with a single copy. Individuals with both mutations have an increased risk of developing CKD, but the mechanism driving this process has been elusive.
Researchers from the University of Leiden, Netherlands, aimed to determine how these mutations drive CKD using kidney organoids. Their work was published in a paper titled, “APOL1 Risk Variants Induce Metabolic Reprogramming of Podocytes in Patient-Derived Kidney Organoids” in Stem Cell Reports.
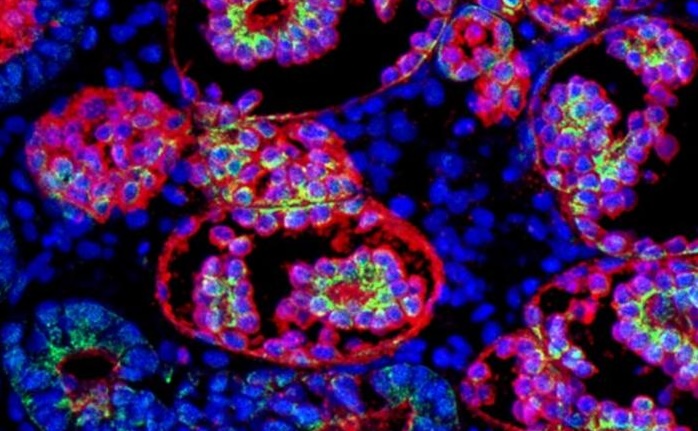
To determine how the genetic risk of these mutations translates into development of CKD, the team developed kidney organoids using induced pluripotent stem cells (iPSCs) from patient skin biopsies from patients with homozygous G1 and G2 mutations to model human apolipoprotein L1 (APOL1)-mediated kidney disease (AMKD).
“We anticipate that this human kidney organoid model will advance our understanding of AMKD and accelerate drug discovery, particularly given that APOL1 is not endogenously expressed in rodents,” Spijker said.
Using CRISPR, they corrected the mutations, generating a control organoid as well to, as the authors wrote, “study disease in a context isogenic to the patient.” Their analysis employed a combination of single-cell transcriptomic, spatial metabolomic data, and fluorescent imaging.
They found that APOL1 mutations have a profound impact on podocytes, resulting in metabolic reprograming. These cells showed a significant reduction in oxidative phosphorylation and tricarboxylic acid (TCA) cycle activity, and upregulation of glycolysis and hypoxia signaling, indicative of mitochondrial dysfunction. Visual analysis showed the podocytes displayed reduced number of mitochondrial branches and shorter branch length.
The effects were increased under inflammatory stress. When organoids were exposed to interferon-gamma (IFN-γ) treatment—a signaling molecule often elevated during infection or autoimmune responses—podocytes with APOL1 mutations exhibited severe mitochondrial impairment and reduced capacity for energy production. This observation may explain why AMKD often manifests or accelerates following inflammatory events, such as viral infections.
The implications of this work extend beyond understanding AMKD. By establishing a reproducible, patient-derived organoid system that captures the molecular inducement and consequences of kidney disease, the study provides a platform for testing therapeutic interventions aimed at restoring mitochondrial function or preventing inflammatory stress responses.
“Overall, the study provides valuable insights that point toward mitochondrial impairment as a central driver of the metabolic reprogramming observed in APOL1 risk variant podocytopathy,” wrote the authors. The conclude that this work is helping to pave the way for future therapeutic strategies for AMKD.