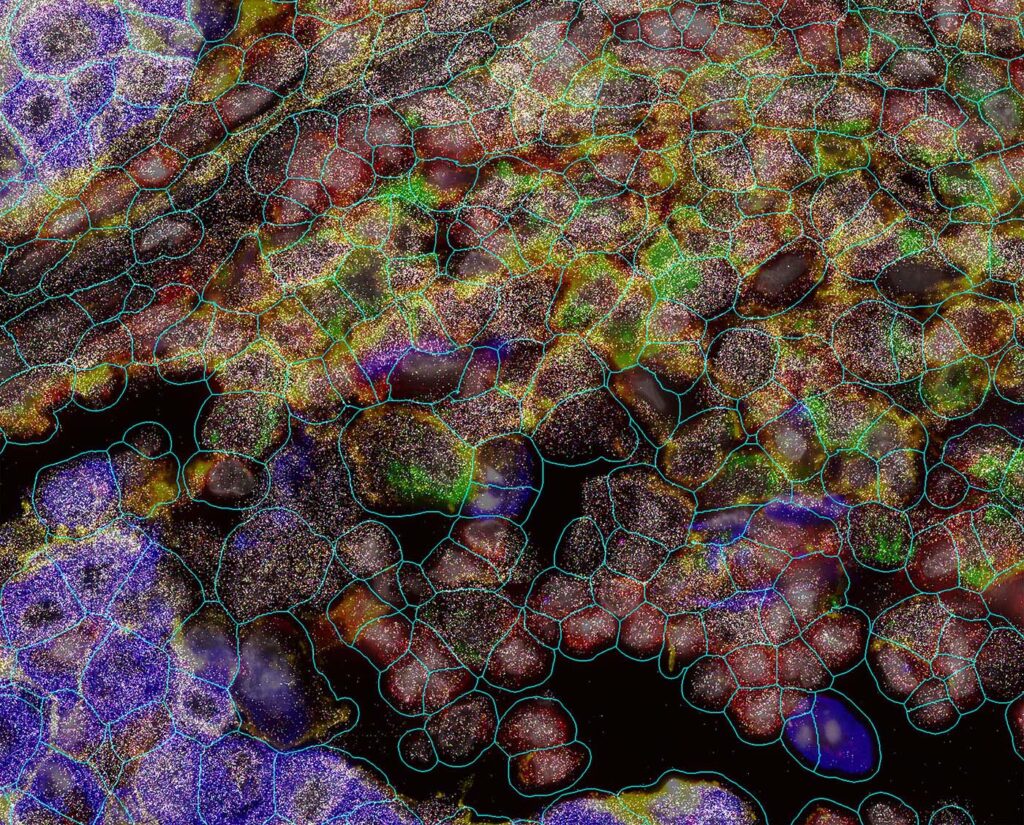
Exploring biology in its native environment is perhaps the ideal scenario for generating better hypotheses about the cellular interactions that influence—and drive—healthy and diseased states, especially in the fields of oncology and neuroscience. The emergence of spatial biology technologies is catalyzing the new insights, permitting 3D transcriptomic and proteomic studies that fill in the spaces in data already accumulated from non-spatial omic applications.
A range of spatial biology tools are available that use thin or thick tissue sections, or punch out select regions of interest for in-depth characterization of FFPE, frozen, or fresh-frozen tissue. The choice of tool depends on the experimental question and the study goals.
The spatial biology field continues to enable deeper insights, higher throughput, and integrated multiomics capabilities, expanding from its initial focus on transcriptomics and select proteomic applications. As more data accumulate, the integration of deep learning algorithms should provide even more knowledge of cellular biology.
GEN talked with several of the scientists gathered at the recent Second American Spatial Biology Congress in June of this year to discuss new technology and findings in this exciting field of life science research.
Volumetric imaging
Targeted 3D multiomics is complementary to whole-transcriptome 2D spatial methods. Together, they form a continuum of discovery. A fully integrated 3D spatial transcriptomics system, the Pyxa™ platform combines STARmap chemistry, volumetric imaging, and proprietary decoding software to generate high-resolution, single-cell gene expression maps within intact 100-micron-thick tissue samples.
STARmap chemistry leverages a dual SNAIL probe design to detect short RNA targets with high specificity and sensitivity, allowing interrogation of transcripts that are traditionally challenging to measure with other in situ methods.
“Instead of reconstructing fragmented information from 5-10 micron-thin 2D slices, tissue architecture is preserved, producing a 3D molecular atlas that reflects the native biological environment,” said Jeremy Lambert, director of product management at Stellaromics. Automated run setup, robust imaging, GPU-accelerated computing, and intuitive data analysis streamline the workflow.
Imaging intact thick tissue samples facilitates the tracing of continuous structures to study rare cell populations and subtle spatial patterns. For example, 3D mapping of the brain has revealed long-range neuronal projections and fine synaptic patterns, and enabled detection of sparse neuronal subtypes and mapping of circuit-level connectivity.
In tumor models, Pyxa has been used to visualize spatially restricted tumor-immune interfaces and gradients of immune infiltration, revealing the organizational principles that govern both normal physiology and disease progression. The ability to profile intact tumor architecture has uncovered microdomains of immune suppression, localized interactions between T cells and cancer cells, and tumor niches that support resistance to therapy.
“Pyxa is positioned to expand beyond RNA into true spatial multiomics, building on published extensions such as detection of actively translating mRNAs, and protein co-detection,” said Lambert. These capabilities open a new frontier for applications where rare events and nuanced spatial biology are critical to understand in their native 3D context.
Incorporating multiomics into platforms will help researchers construct comprehensive 3D molecular atlases that reveal not only which genes are expressed, but also when they are translated and executed at the protein level, within the tissue context.
Immuno-oncology applications
The use of spatial transcriptomics for biomarker discovery and immuno-oncology (IO) pathway interrogation has significant potential. “We are at the beginning of holistically exploring how the spatial signaling that occurs between tumor and immune cells translates into tumor progression,” said Manisha Ray, PhD, director of application development at Vizgen.
In the clinical world, proteomic assays link established clinical modalities and clinical interpretation of biomarkers. “This is why combining spatial transcriptomics and proteomics is so important—the two types of data can together bridge between existing clinical knowledge and emerging mechanistic knowledge,” emphasized Ray.

Transcriptomics, whether spatial or non-spatial, enables analysis of hundreds of genes, up to the whole transcriptome, whereas it has been significantly harder to look at larger numbers of protein targets in a spatial context. The ability to look at dozens of targets to explore immune and tumor pathways and their changes over time can only be accomplished currently by using transcriptomics, and the spatial element is critical.
“But transcriptomics is not everything. Proteomics is not everything. Everything is connected,” said Ray. While transcriptomics and spatial transcriptomics can gather more data than any other method on whole biological programs and pathways, protein addresses the actual function.
“Both the MERFISH Spatial Transcriptomics Method and the InSituPlex® (ISP) assay provide the highest sensitivity proteomics or transcriptomics currently commercially available,” added Ray. The ISP multiplex immunofluorescence method is able to measure up to 12 biomarkers.
“With spatial transcriptomics, for example, you can look at all the immune markers within one MERFISH panel, and the ISP assay provides the clinical link. Not every gene is transcribed at a rate that is useful as a biomarker or is a druggable target,” said Ray.
Combining the insights gained through both methods provides mechanistic information and addressable functional information within the same individual or set of experiments. Initial data, first presented at AACR, are available and will be presented at the upcoming SITC conference.
In addition, the STARVUE™ image analysis platform is in the process of being expanded to take in transcriptomic data. “We will be able to look at mechanistic and functional information at the same time with these advanced analytical techniques,” said Ray.
Imaging the whole transcriptome
To tell a more complete story of every pathway, gene, cell, and transcript in 3D space, the CosMx® Spatial Molecular Imager (SMI) leverages a high-specificity, direct in situ hybridization chemistry to image RNA transcripts from intact tissue sections.
According to Sayani Bhattacharjee, PhD, supervisor of field application scientists at Bruker Spatial Biology, it is the only spatial platform capable of imaging the whole transcriptome at subcellular resolution from a single tissue section to support direct measurement of functional pathway biology.
Critically important is the data accuracy. “CosMx SMI integrates multi-modal cell markers and robustly trained tissue-specific AI models for accurate RNA transcript assignment to individual cells in space,” added Bhattacharjee.
Facilitated by the AtoMx® Spatial Informatics Platform, researchers gain access to the Bruker Spatial Biology cell segmentation suite, study-level and interactive spatial analytics across entire datasets, and flexible data export formats and tools for spatial hypothesis generation, all designed to facilitate open-source spatial data insights.
“Our users are uncovering new findings across a wide breadth of research, ranging from cancer to inflammation to autoimmune conditions to infectious disease to organ transplantation to neurodegeneration,” said Bhattacharjee. Recent discoveries were made through the study of perineural invasion in pancreatic ductal adenocarcinoma, olfactory receptors in inflamed colon, and bacteria-infected metastatic tumor cells.
The future of spatial biology is moving toward deeper insights, higher throughput, and integrated multiomics. Bruker Spatial Biology recently launched CosMx SMI 2.0. The platform allows detection of up to twice the number of RNA transcripts at two times the sample throughput, utilizing the same RNA assays as the initial introductory platform. “This increased sensitivity comes without compromise to signal-to-noise,” Bhattacharjee stated.
CosMx SMI 2.0 also enables simultaneous imaging of up to 76 proteins and whole transcriptome data from the same tissue section for comprehensive same-cell multiomic datasets. Together with GeoMx® DSP’s discovery multiomics with 1200+ proteins and the CellScape™ platform’s differentially quantitative performance in spatial proteomics, Bruker Spatial Biology’s portfolio offers researchers a deep exploration of biology, from regional to single-cell to subcellular insights.
A new approach to cell sorting
A new technology that allows physical isolation of precisely defined cellular populations, while preserving their native spatial context, gives researchers the ability to perform comprehensive molecular characterization of spatially defined cellular populations.

SLACS (Spatially-resolved Laser Activated Cell Sorting) uses a low-energy IR pulse laser to specifically dislodge target cells from fixed tissue, while preserving RNA integrity and spatial coordinates. CosmoSort™, a cell sorter powered by Meteor Biotech’s SLACS technology, integrates high-resolution imaging, AI-driven target selection, and rapid IR laser-based sorting.
The FFPE, frozen, or fresh-frozen tissue sample is mounted on a proprietary CosmoSlide and stained to visualize target cells. AI-driven software facilitates imaging and region of interest (ROI) selection. Next, the IR laser “punch” isolates targets. Upon impact, the slide’s specially coated layer vaporizes at the targeted spot, releasing the target cells directly into a 96-well retrieval plate. Downstream analysis supports full-length RNA sequencing and other omics assays.
“CosmoSort is fast—about one second per isolation—which is critical for minimizing contamination and degradation,” said Sumin Lee, PhD, CTO, Meteor Biotech, The IR laser avoids charring and preserves surrounding tissue.
Meteor Biotech developed the SLACS platform to physically isolate and molecularly characterize specific cellular populations, while maintaining spatial context. As the company likes to say, “what you see is what you get.”
The resulting tool helps researchers selectively analyze biologically relevant regions, without processing entire tissue sections, significantly improving analytical efficiency and reducing sequencing costs. Lee added that, “The paradigm shift from observation to isolation enables full-length transcriptome recovery and multi-modal molecular analyses previously unattainable.”

The CosmoSort platform has gained traction in academic and pharmaceutical research, with applications that include oncology research, neuroscience, rare cell biology, organoid studies, and translational research.
In the future, Lee predicts that imaging and molecular profiling will achieve subcellular resolution at scale and fully integrate multiomics for simultaneous transcriptome, proteome, and epigenome profiling from the same spatial coordinates. Implementation of deep learning-based cell segmentation and classification systems will enable automated identification of complex cellular phenotypes and morphological states.