Despite its outsized impact on fertility and disease risk, the ovary’s biology has remained understudied. Researchers charted its aging in unprecedented detail, pointing to ways to slow the process.
Image credit:Diana Laird
There’s an organ in human bodies that ages twice as fast as other tissues and becomes completely dysfunctional by the time a person hits their early fifties: the ovaries.1 “If I told you that any other organ in your body was going to function one fifth as well when you were 40, than it did when you were 25, you would be really upset,” said Diana Laird, a reproductive biologist at the University of California, San Francisco. “Why aren’t we upset about this with our ovaries?”
The ovaries are two oval-shaped reproductive glands that release eggs and produce hormones such as estrogen and progesterone. Menopause occurs once these organs stop performing their functions or if they are surgically removed. While this transition leads to the expected cessation of fertility, the lack of the associated hormones puts an individual at higher risk for conditions like osteoporosis, cardiovascular diseases, and urinary tract infections.2 Despite the wide-ranging effects of ovarian aging, there’s an incomplete understanding of the cellular and molecular drivers of the process.
Researchers have relied heavily on rodent models to study ovarian aging. However, they have struggled to translate these findings to the clinic because it’s not well understood how ovarian aging compares in rodents and humans. “We should really be studying the ovaries of mice and humans together to understand whether mice could be a reasonable model system for understanding how the ovary ages and trying to slow down that aging and manipulate it,” Laird said.
Now, Laird and her colleagues have generated a comprehensive reference of ovarian aging in mice and humans.3 It not only highlights the decline in the egg numbers but also describes the changes to the surrounding cellular microenvironment. Their findings, published in Science, lay the foundation for extending women’s fertility and consequently improving their health.
“This is a model par excellence. We don’t have any better models in the laboratory yet,” said Yousin Suh, a reproductive geneticist at Columbia University who was not involved in the study. “The question now is how do we best utilize this atlas to improve women’s health through manipulation of ovarian function.”
To understand how a normal ovary ages over the years, Laird and her team decided to examine the intact organs. However, the large size and the irregular composition of the ovaries posed a technical hurdle. “Simply slicing the ovary into histologic sections and counting the number of eggs is really labor intensive and doesn’t necessarily represent the full extent of what’s happening throughout the organ,” Laird said. To overcome this, she and her team developed a 3D-imaging technique wherein they optically cleared intact ovaries and visualized their insides using light sheet microscopy. Using this method, the scientists first examined mouse ovaries from two- to twelve-month-old mice, a window that corresponds to humans between the ages of 20 and early 40s. In older mice, equivalent to 30- to 40-year-old humans, Laird and her team observed a steep decline in the number of eggs undergoing changes for ovulation and in those waiting to mature. The success rate of conception with in vitro fertilization also plummeted as the animals grew older.
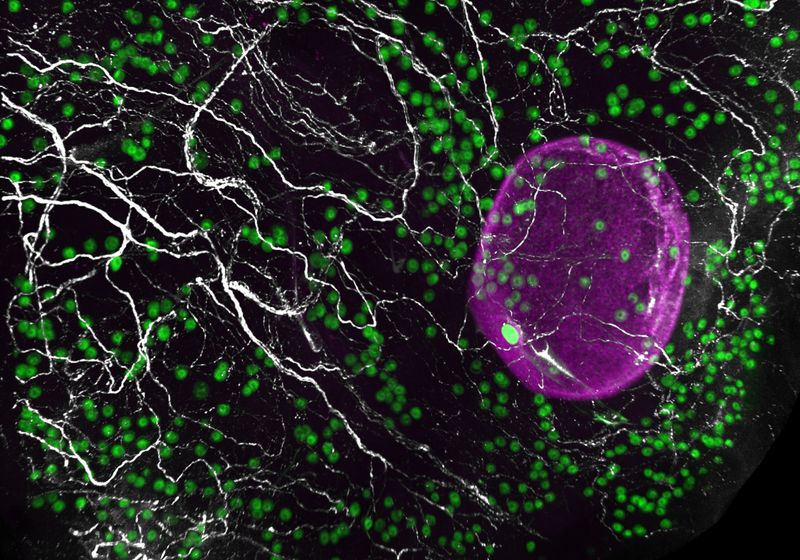
The number of eggs (green and magenta) declines as the ovary ages.
Diana Laird
To study this developmental change in humans, the team obtained donated ovaries from victims of sudden death who were either in their early (23 to 30 years old) or late (37 to 58 years old) reproductive ages. To their surprise, human eggs were present in pockets, contrary to the uniform distribution observed in mouse ovaries. The density of eggs within these pockets declined with age.
Next, the team analyzed the various cells surrounding the eggs using single-cell RNA sequencing. Upon examining close to 100,000 cells from mouse and human ovaries, Laird and her colleagues identified 11 different cell types, such as fibroblasts, epithelia, and theca, among others. They also found an unexpected cell type—glia—which is normally associated with neurons.
Considering this surprising finding, the team analyzed the ovaries using neuronal markers and observed that sympathetic nerves form intensely branched networks intertwined with the ovarian cells. The team could detect them as early as eight weeks post conception in humans and at early embryonic stages in mice.
Researchers have previously shown that sympathetic nerves influence egg maturation cycles, especially in the context of polycystic ovarian syndrome.4 To study this in more detail, the team generated a mouse model without any sympathetic nerves and observed that these animals had fewer growing eggs and more immature eggs than their healthy counterparts, indicating a role for the nervous system in ovarian health.
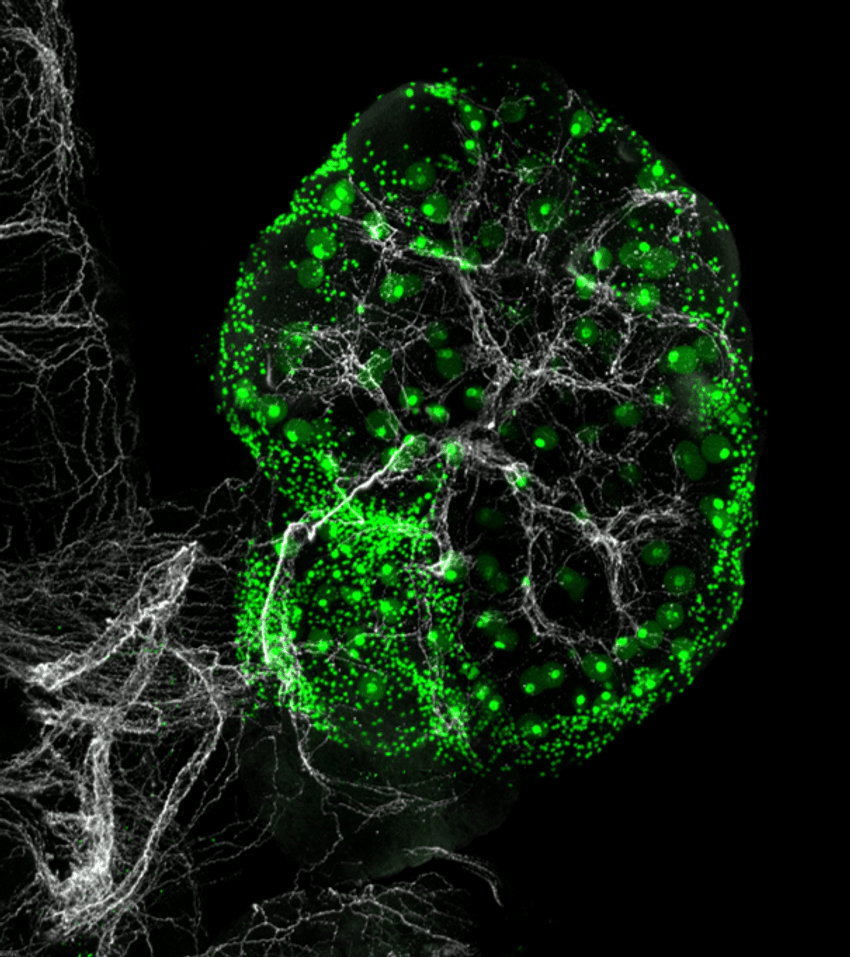
Sympathetic nerves (white) innervate ovaries in humans and mice, regulating egg (green) maturation.
Diana Laird
“This opens up critical avenues of intervention to improve ovarian function and influence women’s overall health,” Suh said.
Lastly, the researchers noticed that fibroblasts, smooth muscle, and epithelial cells exhibited the greatest age-related transcriptional changes across species, suggesting that anti-fibrotic interventions might preserve ovarian function during aging.
Going forward, Laird hopes to model ways to slow aging of the ovary in mice and progress to primate studies and trials in humans.
“We’re really thrilled that our work on female reproductive aging is being recognized by the scientific community and by the public as being important,” Laird said. “This gives us hope that we can make some progress in our lifetime toward recognizing that reproductive health is not something that we should only think about when we’re trying to reproduce.”