Jonathan D. Grinstein, PhD, North American Editor of Inside Precision Medicine, hosts a new series called Behind the Breakthroughs that features the people shaping the future of medicine. With each episode, Jonathan gives listeners access to his guests’ motivational tales and visions for this emerging, game-changing field.
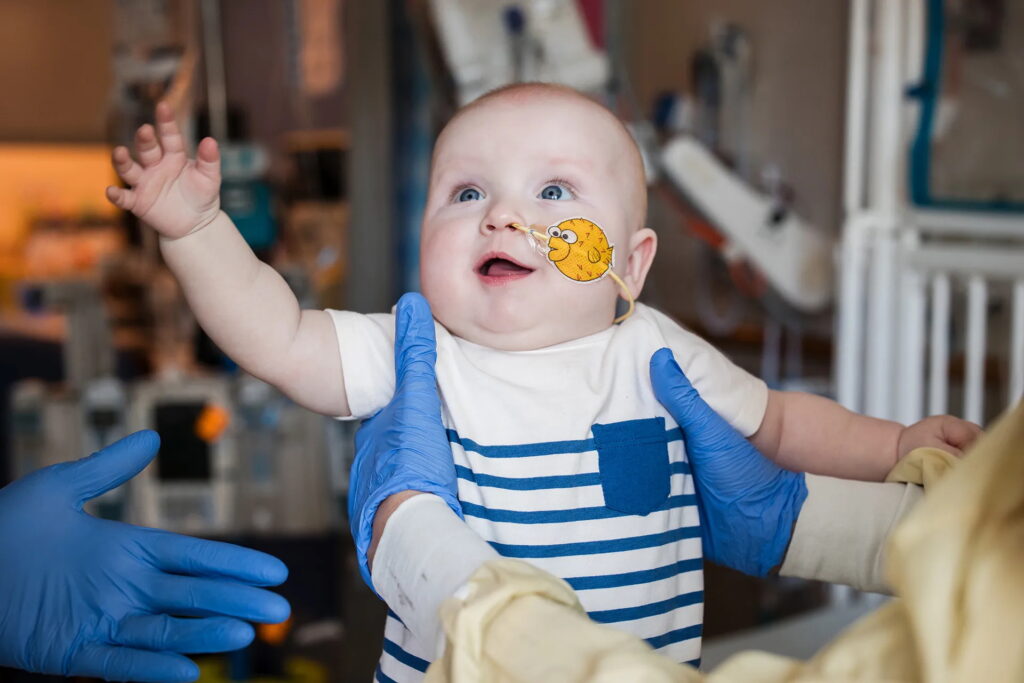
When a rare genetic mutation left baby KJ without a viable treatment option, scientists and regulators faced an ethical and scientific crossroads: should medicine move fast enough to save a single child? Vanessa Almendro-Navarro, PhD, saw in KJ’s case both an urgent humanitarian mission and a glimpse of medicine’s future. Her team developed a customized gene-editing therapy designed specifically for KJ’s unique mutation—a process that would have been unimaginable just a few years ago.
In this interview, Almendro-Navarro discusses how the experimental treatment came together, what it reveals about the new frontier of one-patient medicines, and the profound challenges of balancing speed, safety, and ethics. She reflects on the unprecedented collaboration among families, regulators, and researchers that made KJ’s therapy possible—and what it might mean for the next generation of children with ultra-rare diseases.
This interview has been edited for length and clarity.

IPM: How was Danaher able to develop a personalized gene-editing medicine for Baby KJ?
Almendro-Navarro: The story of baby KJ is really fascinating. KJ Muldoon, in the very early days, was already starting to show signs of lethargy. Kiran Musunuru, MD, PhD, and Becca Ahrens-Nicklas, MD, PhD, were the doctors treating KJ. Kiran and Becca were able to diagnose urea cycle disorder (UCD) with a very specific mutation that happens to be one of the most severe in terms of prognosis mutations for UCD. Kiran and Becca had been planning for many years already how they would go about developing a personalized gene editing treatment, so that the moment they identified this patient, they knew what was going to be that development path. They just needed to have the right partners.
Kiran happened to pick up the phone and call Fyodor Urnov, principal investigator of our Danaher beacon with the Innovative Genomics Institute (IGI), and said, “I identified this patient. He is eligible for a base editor.” We think we can do it. Fyodor picked up the phone and called Sadik Kassim, the chief technology officer at Danaher Corporation. In 15-20 minutes, basically, the team was assembled. There was this ability to quickly assemble a team that collaborated in an unprecedented manner to develop a treatment in six months. Beautiful. We did it. We developed a treatment with your KJ Muldoon.
We want is to make sure that this doesn’t become just an anecdote in the field. We’ve demonstrated the technical feasibility of being able to do this, and that journey allows us to see what needs to be true for us to be able to scale these personalized gene editing technologies. And it’s not going to be a journey without challenges, but the opportunity for us now is to ensure that, again, newborn sequencing can be standardized to be able to identify these babies that need these treatments as rapidly as possible. We need to have the right network so that these patients can be referred to the right interventional genomic clinics.
We need to be able to have the right technologies to design, because we are not developing drugs. We are designing drugs over and over again for different mutations for patients, qualifying those treatments in a way that is aligned with regulatory requirements and ensuring the safety for the patient, being able to deliver and monitor the response in the patient, and most importantly, building a reimbursement model through Medicaid or from private payers. These treatments need to be paid for. We need to connect all the dots.
With newborn sequencing, we’re able to identify the patients, but if we can do nothing about it, it’s not going to deliver any impact to the babies. We need to streamline every single point of innovation along the way. This is going to require a fundamental shift in the way that we consider personalized genomic interventions. It’s very different from the traditional therapeutic development. These are personalized interventions, and maybe we need to start looking at them that way—as interventions, not treatments. Just like cardiology has interventional cardiology clinics, we’ll have interventional genomics clinics in the future.
IPM: How do personalized gene-editing therapies become the clinical standard?
Almendro-Navarro: The future could be a place where we can choose what’s the best tool for that genomic intervention. We did base editing in Baby KJ. There are traditional CRISPR gene editing technologies out there that have demonstrated clinical efficacy and sensitivity, of course. There are antisense oligonucleotides. We are doing RNA editing today. The way for us to intervene with genetic tools is expanding significantly. What we need in the future is being able to fully understand how to make a much smarter choice of that genomic intervention based on the modification that you want to do and that will depend again on the mutation and might depend on the patient, the age of the patient and many—there might be many considerations that go into choosing that genomic tool for the treatment you want to do.
For this potential future to happen, especially within the concept of personalized treatments, the only way that we are going to be able to choose the right tool for the right mutation is by understanding these technologies, and we cannot do that unless there is data sharing at the individual patient level. One Baby KJ cannot inform a full population. We are going to need to explore additional treatments in additional patients. By having that patient-specific data, we can identify the markers and rules for matching the right patient to the right genomic intervention.
IPM: What will it take for gene-editing therapeutics to go from treating rare diseases to mass, chronic indications?
Almendro-Navarro: This is not going to be much different than how we think about mass or chronic indications in larger populations. It’s about being able to have access to the data and use it to identify the right patterns of who the best responders are for certain treatments. We need to have access to the data. Now that we have demonstrated in KJ Muldoon, we need to make sure that we all become much more thoughtful about data sharing so that we can start building that knowledge in a federated model on those individualized, personalized cases to really infer and learn from the full population.
The use of patient-surrogate models was a crucial factor for us regarding Baby KJ, given the lack of time to develop many preclinical models. We knew the sequence, which was incorporated into traditional cell lines and used in a traditional animal model. One of the most important things they sought was to ensure that the genotoxicity package for this specific base editor was acceptable for the treatment we aimed to develop. That was a personalized genotoxicity assessment in surrogate patient models. There is a lot to learn from surrogate models, but what’s important for us is to do long-term monitoring of Baby KJ and learn from that efficacy, hopefully in the coming years. That’s going to be important. But it’s all about data sharing. That’s going to be crucial for us to organize the structure and enable it for the benefit of all interventional geneticists who will be working on this.
IPM: How will personalized gene editing treatments become equally accessible?
Almendro-Navarro: If personalized gene editing treatments are going to be considered genomic interventions administered at interventional genomic clinics, we will need to build a network by which hospitals that are doing newborn sequencing, when they identify a patient, have that awareness of that network to refer these patients to those particular interventional clinics. That’s a future that we envision. Then what’s the cautionary tale that we need to have here, and where is it going to be important for us to be mindful about, that before we get there, before we start running because of the promise of what we can deliver, it’s going to be that we are still far away. These are early days; we’ve done one baby—we need to make sure that we continue to reproduce this model and that we really understand how to standardize these platforms. We need to ensure that these genomic interventions are seen as a reliable, novel therapeutic approach, and reliability is going to be critically important to build as well as that trust in the regulators and trust in the payers. We are working on that; that’s the first step.
Once we have developed additional treatments for this UCD and demonstrated that the platform is solid and robust and that we understand what makes those treatments successful and what patient characteristics make them ineligible for these treatments, we will be able to scale and proceed to the next indication. There is still some clinical validation and reliability in the platform that we will need to build. But the future that we envision has the end-to-end ability to deliver these treatments. The bottleneck within the healthcare system is not going to be who pays for the treatment. The bottleneck will be the capacity of those interventional clinics to treat all the babies that are diagnosed in the U.S. That’s the mindset shift and operational shift that hopefully we’ll see in the years to come.
IPM: What do you believe is an underdeveloped but critical component to making personalized gene-editing therapies available?
Almendro-Navarro: We are very interested in this concept about patient journeys with longitudinal data from healthcare records and using that to start inferring paths for individuals. This relates to whole genome sequencing, and only a tiny percent of the genome today has clinical significance. There are so many single nucleotide polymorphisms (SNPs) and so many variants that we don’t yet know what the clinical significance of those variants is. How do we start connecting the dots and understanding what is driving your healthcare journey? I think that’s going to be the next iteration of where we are going to be moving next.
Whole genome sequencing costs $100. We need to start understanding what we are going to be doing with that information. How can we pair those genomic, epigenomic, proteomic, and biochemical profiles to understand really what that would look like in our healthcare or health trajectories in the future? I’m extremely fascinated.
The opportunities that come with all of the progress that we are making with data and artificial intelligence, as well as our ability to interrogate multiple data points ranging from omics data to healthcare interventions to lifestyle to even your work patterns, play an important critical role in determining whether or not you are predisposed to disease. Today we have the right tools that allow us to start gathering all that disparate data and try to make sense of it to predict those health trajectories. This is an area that excites me significantly, and I’m hoping that in the future, we are going to start really uncovering how we can make better life choices to direct our health trajectory towards better outcomes.